DUPIXENT® coverage criteria on Saskatchewan formulary (Exception Drug Status [EDS])19
To apply for EDS, authorized prescribers will be required to submit a request by telephone, mail or fax.
For the treatment of moderate-to-severe1 atopic dermatitis in patients 12 years of age and older who:
• Are inadequately controlled with topical prescription therapies, or where these treatments are not appropriate, AND
• Have had an adequate trial with an inadequate response, or were intolerant, or are ineligible for each of the following therapies: phototherapy (where available), methotrexate and cyclosporine.
Requests must include documentation of the Eczema Area and Severity Index (EASI) score and Physician Global Assessment score.
Initial approval: Six (6) months.
Renewal criteria
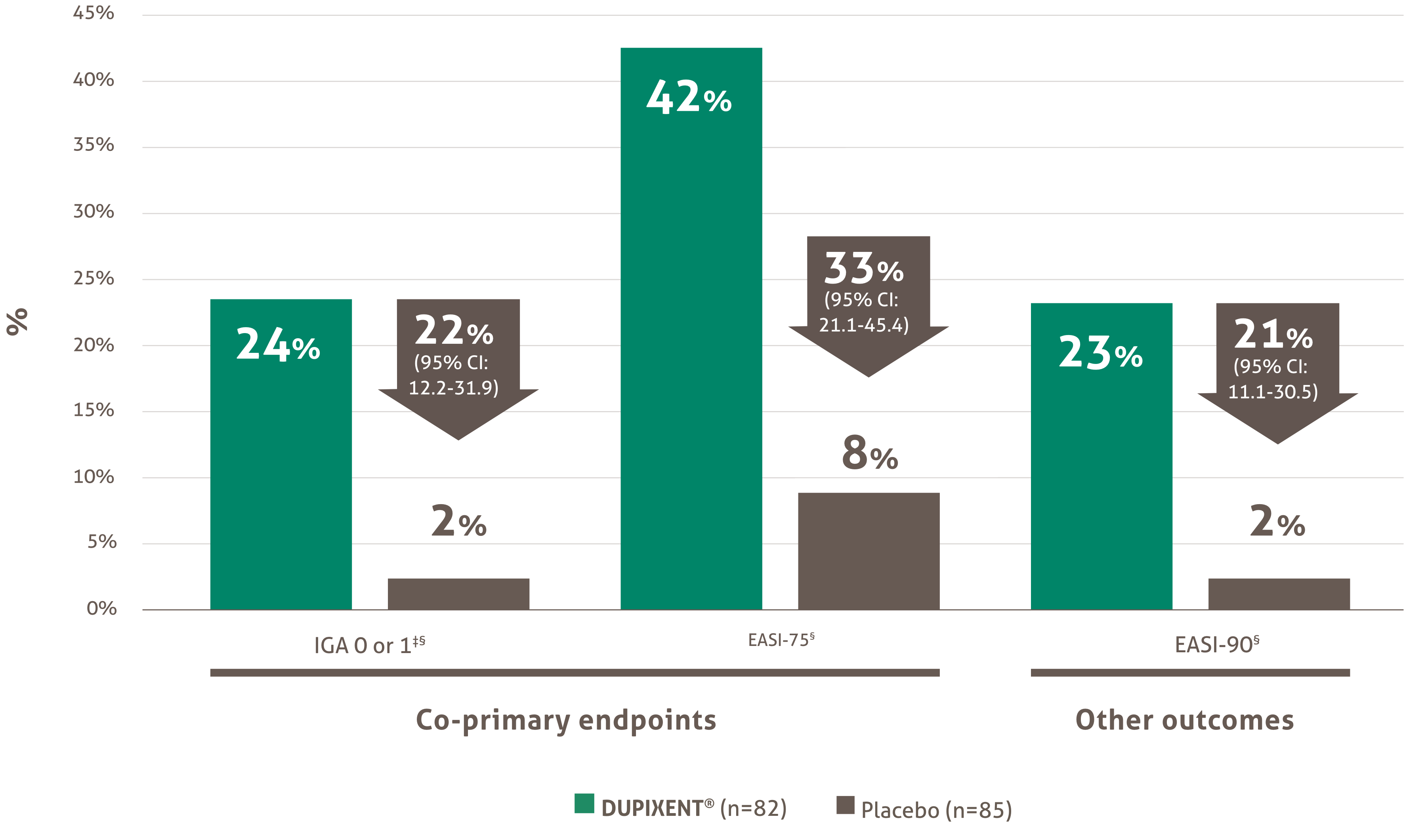
Renewal requests will be considered for patients where there has been a 75% or greater improvement from baseline in the EASI score (EASI-75) six months after treatment initiation and where this response is maintained thereafter every six months.
Renewal requests must include recent EASI score.
Renewal approval: Six (6) months.
Requests for coverage for this indication must be made by, or in consultation with a dermatologist.
1 Moderate-to-severe atopic dermatitis is defined as an EASI score of 16 points or higher; or a Physician Global Assessment score of 3 or 4.
DUPIXENT® coverage criteria through Manitoba Drug Benefits (Exception Drug Status [EDS])20
To submit an EDS request, authorized prescribers will be required to fill out a form.
For the treatment of atopic dermatitis only if the following conditions are met:
Initiation Criteria
• Patients aged 12 years and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
• Patients must have had an adequate trial or be ineligible for each of the following therapies: phototherapy (where available), methotrexate, and cyclosporine.
• Patients who have had an adequate trial of phototherapy, methotrexate, and/or cyclosporine must have documented refractory disease or intolerance.
• The physician must provide the Eczema Area and Severity Index (EASI) score and Physician Global Assessment score at the time of initial request for reimbursement.
• The maximum duration of initial authorization is six months.
Renewal Criteria
• The physician must provide proof of beneficial clinical effect when requesting continuation of reimbursement, defined as a 75% or greater improvement from baseline in the EASI score (EASI-75) six months after treatment initiation.
• The physician must provide proof of maintenance of EASI-75 response from baseline every six months for subsequent authorizations.
Prescribing Conditions
• The patient must be under the care of a dermatologist.
• Dupilumab is not to be used in combination with phototherapy or immunosuppressant drugs, such as methotrexate or cyclosporine.
EASI: Eczema Area and Severity Index
DUPIXENT® coverage criteria in Ontario through the Exceptional Access Program (EAP)21
To submit an EAP request, authorized prescribers will be required to fill out a form.
Initiation criteria
DUPIXENT® is being used to treat moderate-to-severe atopic dermatitis in a patient who meets the following criteria:
1. is 12 years of age or older; and
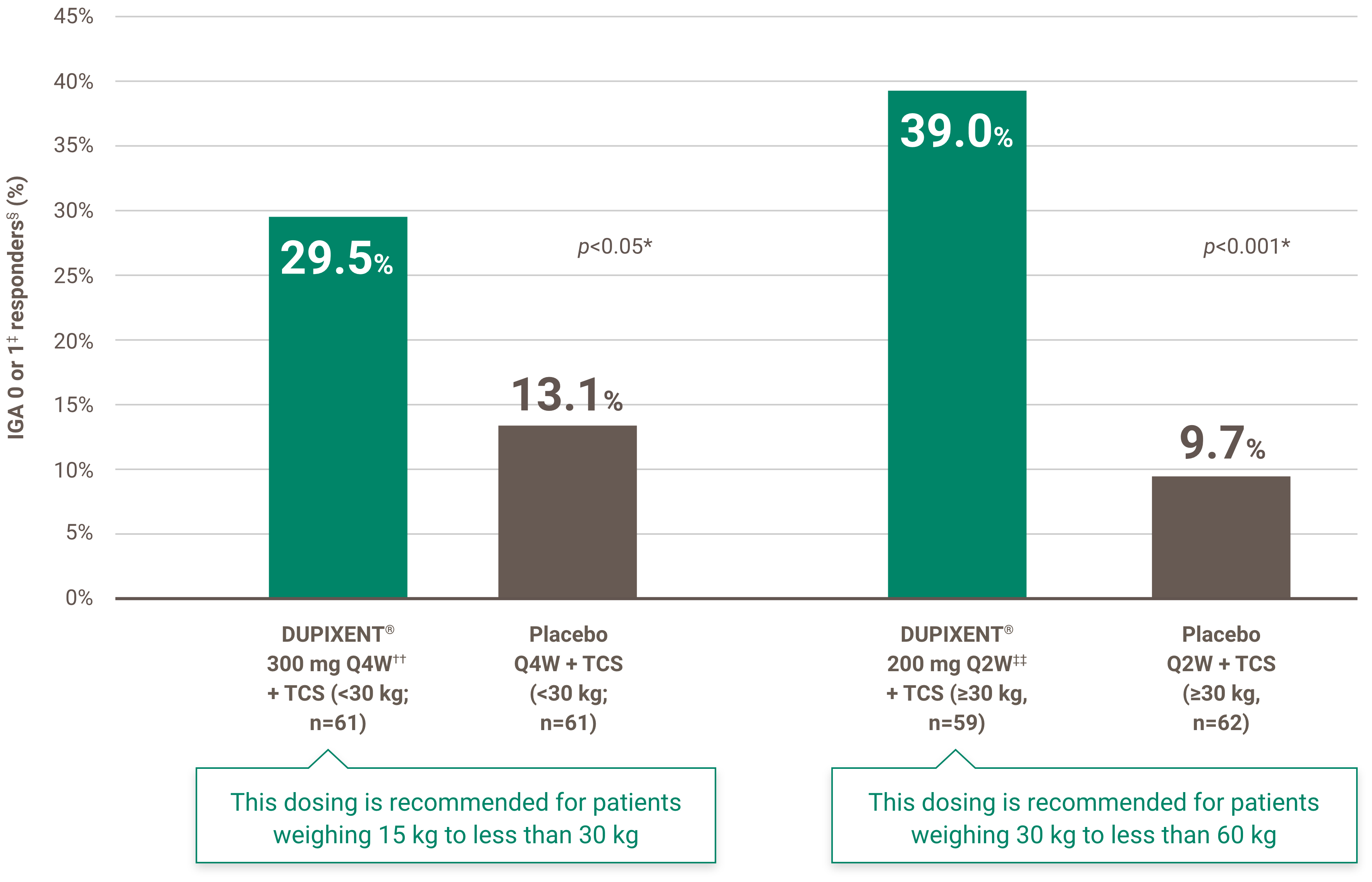
2. has been diagnosed with moderate-to-severe atopic dermatitis as defined by an Eczema Area and Severity Index (EASI) score equal to or greater than 16 points; and
3. has experienced failure to maintain adequate control of their dermatitis with topical prescription therapies (if these therapies are not advisable, the authorized prescriber must provide reasons why they could not be used with the EAP request); and
4. has experienced failure or intolerance with an adequate trial of phototherapy (where available), methotrexate, and cyclosporine;1 and
5. DUPIXENT® is prescribed for the patient by a dermatologist, pediatrician, or immunologist; or in consultation with one of these specialists and the authorized prescriber includes the consult note with the EAP request.
Exclusion Criteria: (Patients meeting any of the following will not be funded)
• DUPIXENT® will not be funded if it is being used in combination with phototherapy or immunosuppressant drugs (e.g., methotrexate, cyclosporine)
• DUPIXENT® will not be funded in combination with other biologics used for atopic dermatitis.2
Notes:
1 For each treatment used, provide documentation of refractory disease and/or intolerance (including a description of the adverse effect and severity of reaction). If a patient is deemed to be ineligible or contraindicated to receive the treatment, provide the reason(s) for their ineligibility.
2 The concurrent use of dupilumab used in combination with other biologics used for other conditions will be considered on a case-by-case basis.
Renewal criteria
First renewal:
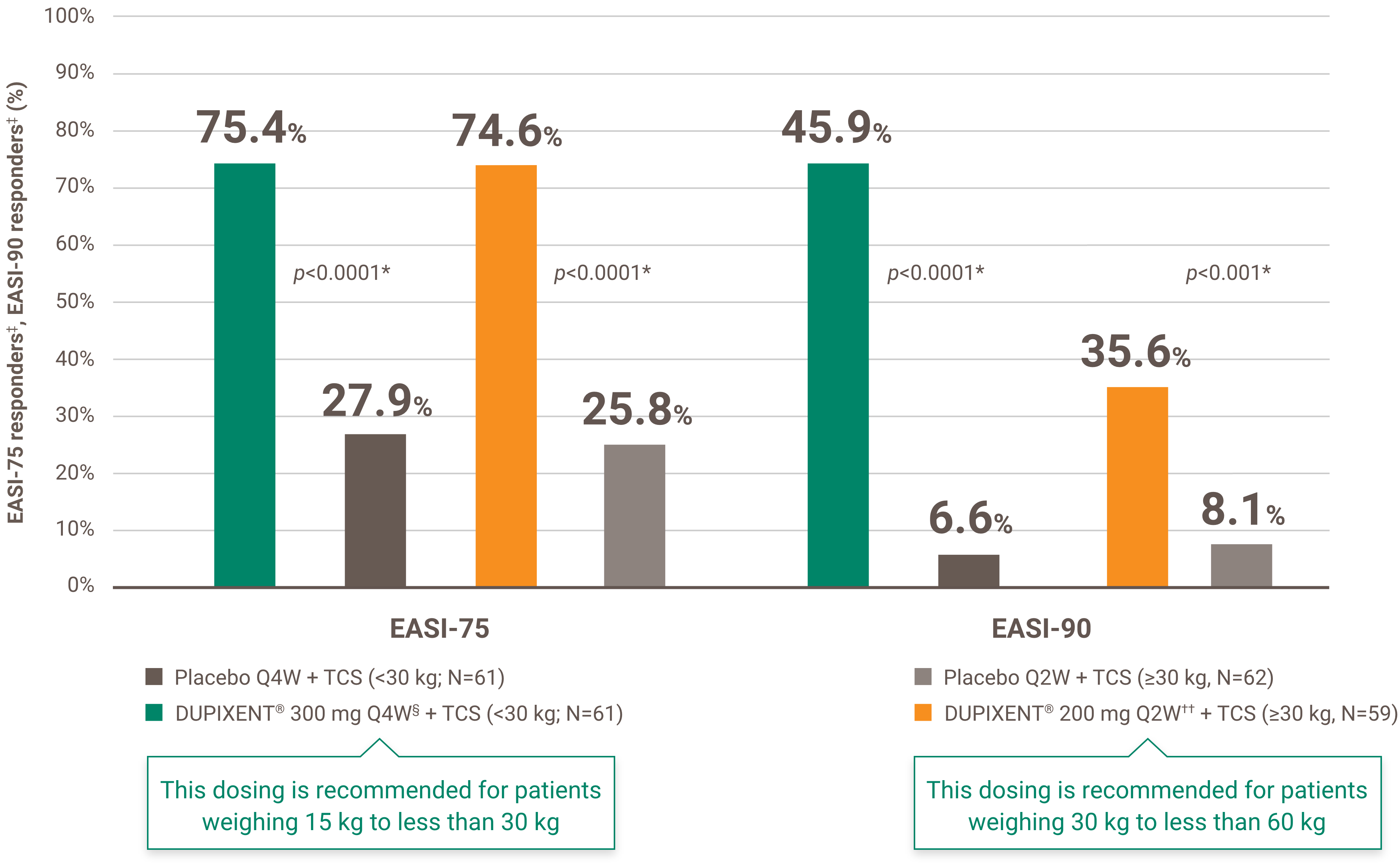
Renewal of funding will be considered in patients with documentation of benefit from treatment. Benefit from treatment is defined as a 75% or greater improvement from baseline in the Eczema Area and Severity Index (EASI) score (EASI-75) within six months of treatment initiation.
Subsequent renewals:
Subsequent renewal of funding will be considered in patients who maintain the 75% or greater improvement in EASI-75 response from baseline.
Duration of Approval for initial requests: 6 months
Duration of Approval for first and second renewal: 6 months
Duration of Approval for 3rd and subsequent renewals: 1 year
DUPIXENT® coverage criteria in Quebec on the RAMQ* list of exceptional medications22
For the treatment of patients aged 12 years and over suffering from a moderate-to-severe form of chronic AD:
- in the presence of a score ≥16 on the EASI or where the face, palms, soles or genital area are severely affected; and
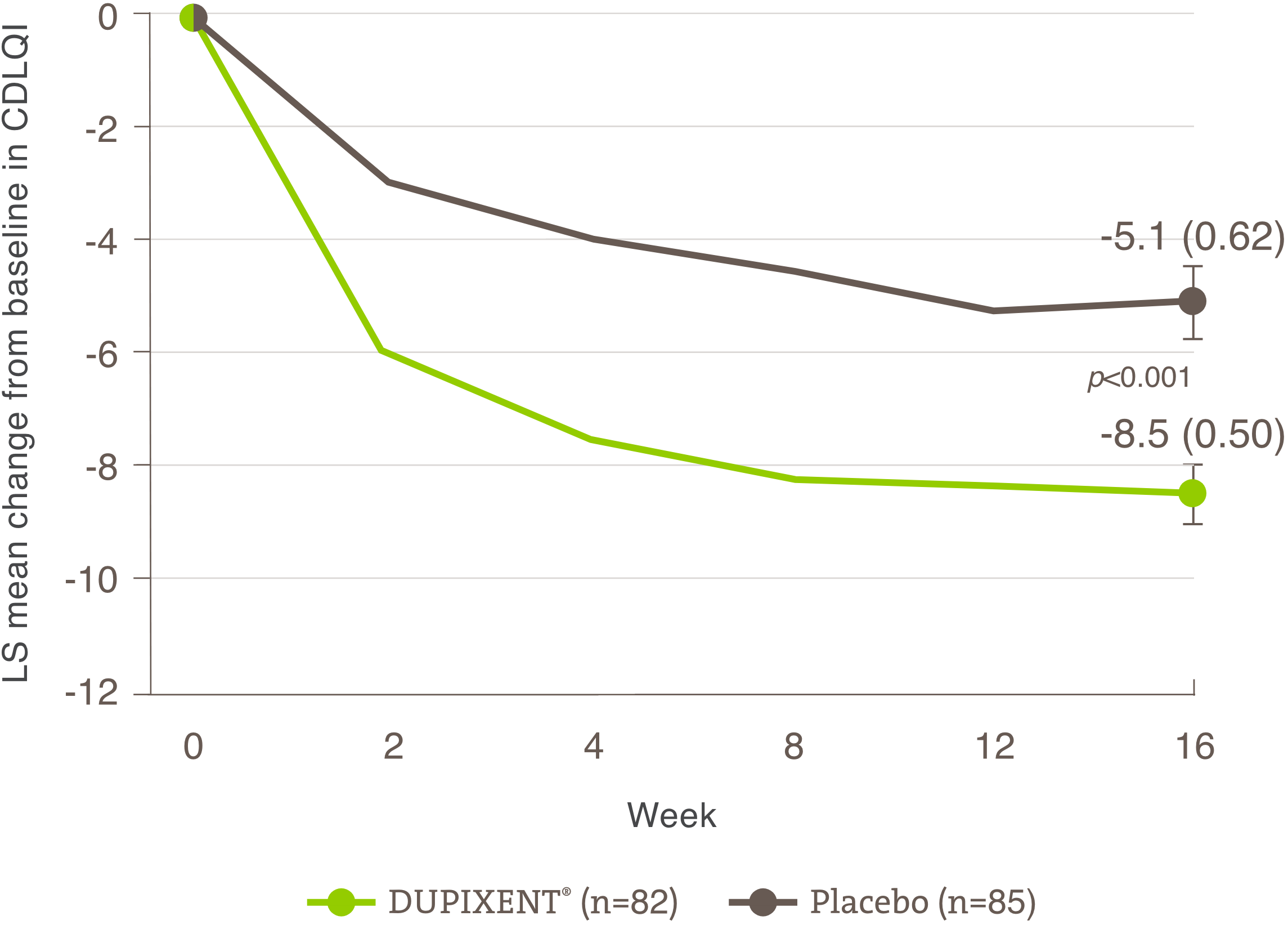
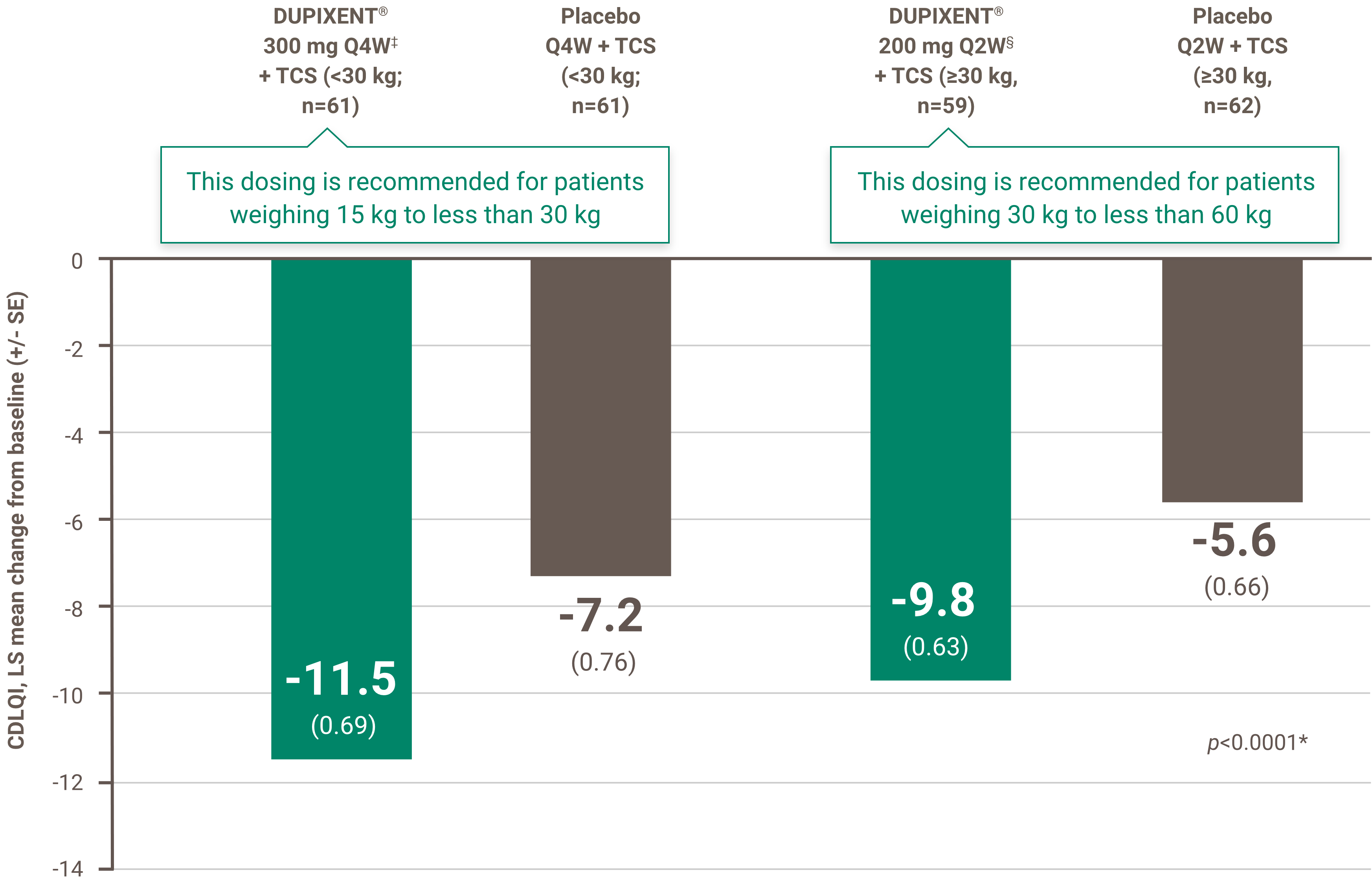
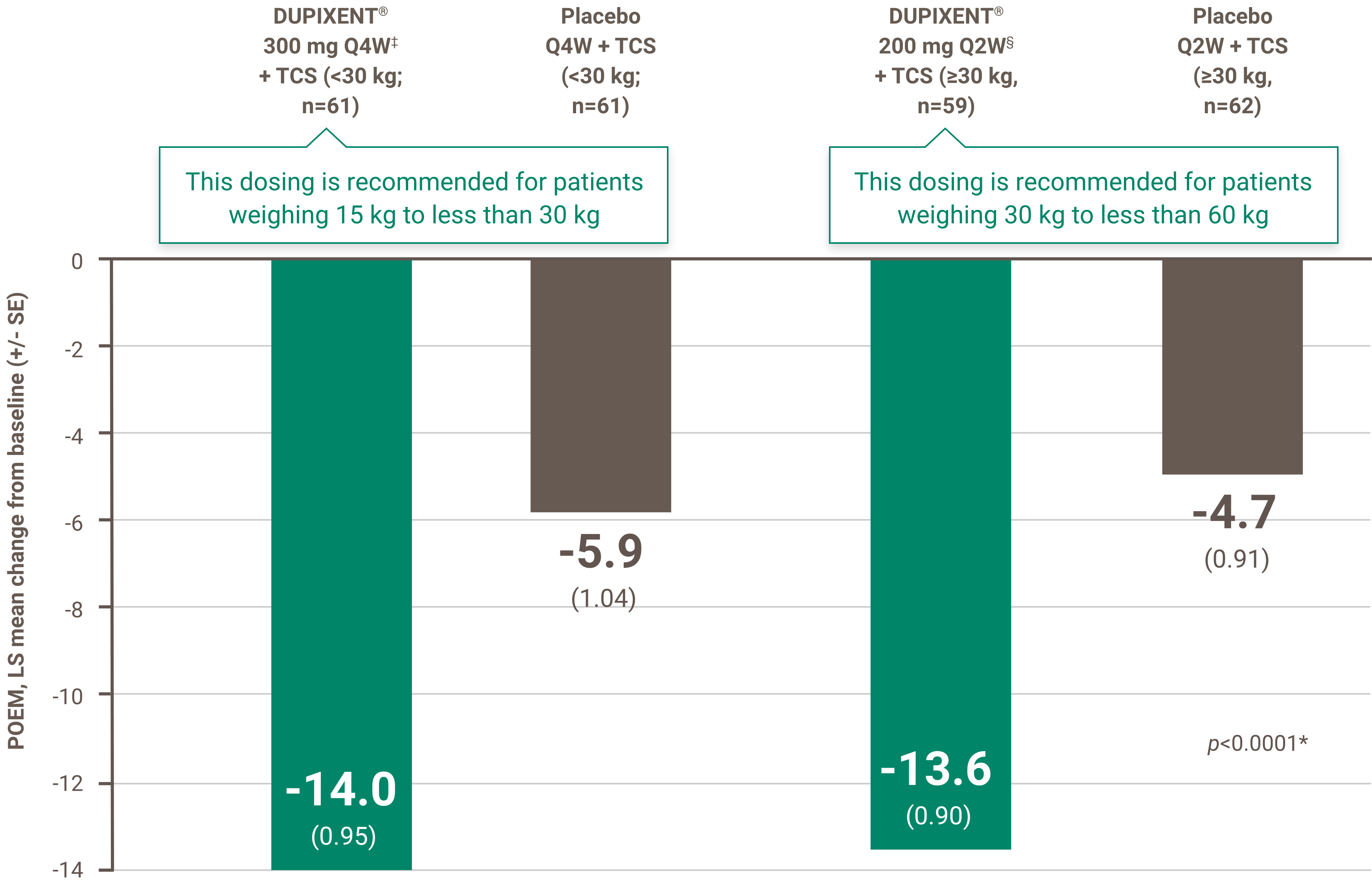
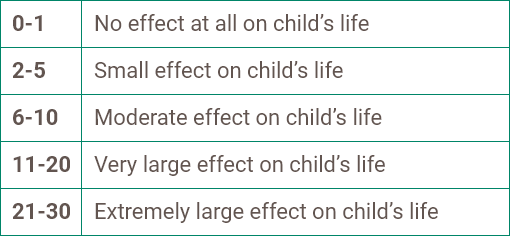
- in the presence of a score ≥8 on the DLQI or CDLQI; and
- where 10% or more of the body surface area is affected, except in cases where the face, palms, soles or genital area are severely affected; and
- where the disease is insufficiently controlled despite the use of topical treatments including at least two medium- or high-potency topical corticosteroids and one topical calcineurin inhibitor; and
- where a phototherapy treatment of ≥30 sessions during three months has not made it possible to optimally control the disease, unless this treatment is contraindicated, not tolerated or not accessible, or where a treatment of ≥12 sessions during one month has not provided significant improvement in the lesions.
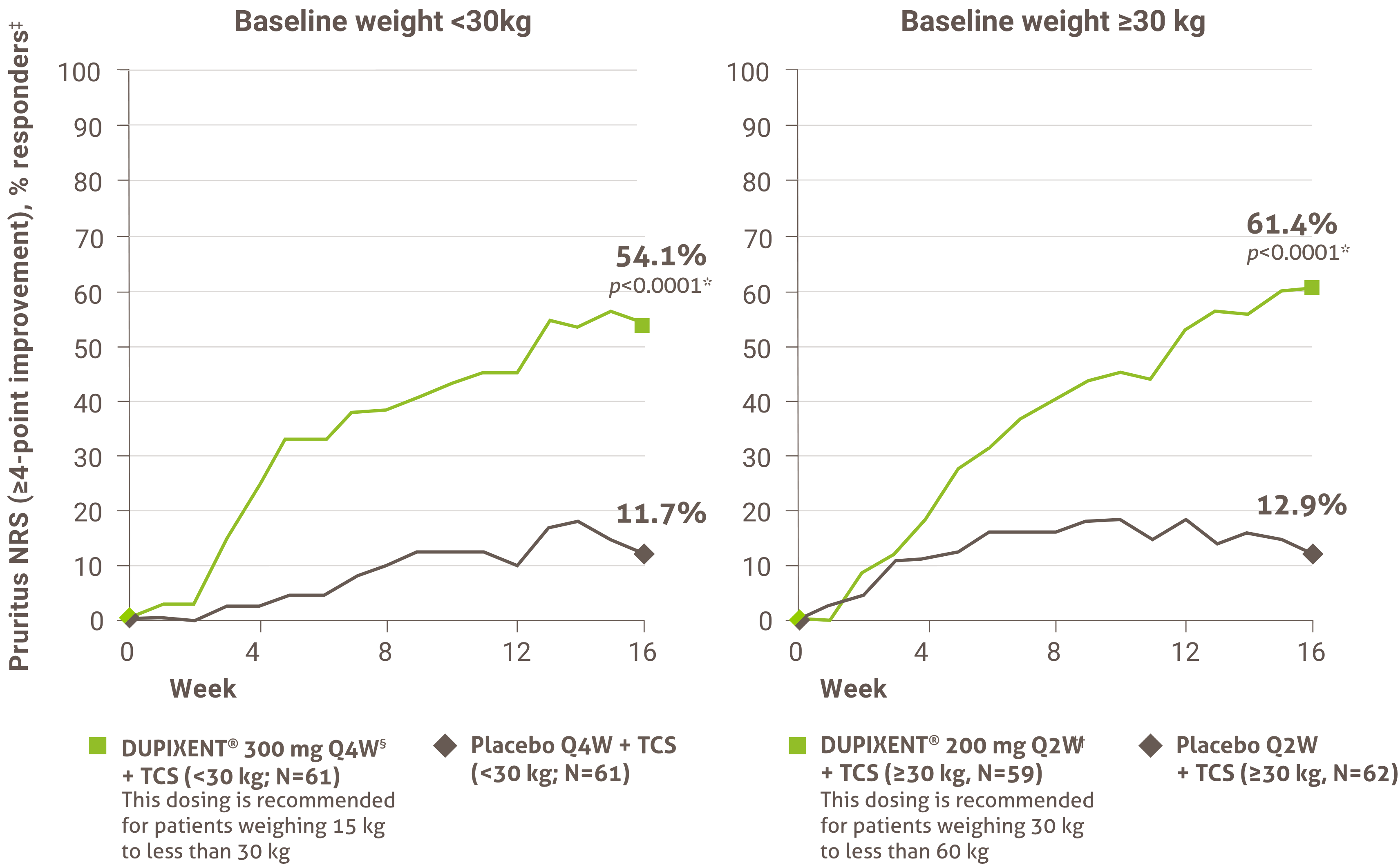
The initial request is authorized for a maximum period of four months. Upon subsequent requests, the physician must provide information making it possible to establish the beneficial effects of the treatment, specifically: an improvement of at least 75% in the EASI score compared to the basic level; or an improvement of at least 50% in the EASI score and a decrease of at least five points on the DLQI or CDLQI questionnaire compared to the basic level; or significant improvement of lesions on the face, palms, soles or genital area compared to the pre-treatment assessment and a decrease of at least five points on the DLQI or CDLQI questionnaire compared to the basic level.
Requests for continuation of treatment are authorized for a maximum period of 12 months. Authorizations for dupilumab are given for a maximum initial dose of 600 mg followed by a maximum dose of 300 mg every two weeks.
* Official mark of the Régie de l’assurance maladie du Québec.
DUPIXENT® coverage criteria on New Brunswick Drug Plans (special authorization)23
To request coverage through special authorization, authorized prescribers will be required to fill out a form.
For the treatment of moderate-to-severe atopic dermatitis in patients 12 years of age and older who meet all of the following criteria:
• Refractory or have contraindications to an adequate trial of topical prescription therapies
• Refractory, intolerant or have contraindications to an adequate trial of phototherapy (where available), methotrexate, and cyclosporine
• Baseline Physician Global Assessment score of 3 or greater and Eczema Area and Severity Score of 7.1 or greater
Renewal Criteria
• Requests for renewal must provide proof of beneficial clinical effect defined as a 75% or greater improvement from baseline in the Eczema Area and Severity Index (EASI-75) score six months after treatment initiation.
• Proof of maintenance of EASI-75 response from baseline must be provided for subsequent authorizations.
Clinical Note:
• Not to be used in combination with phototherapy or immunosuppressant drugs (e.g., methotrexate, cyclosporine).
Claim Notes:
• Must be prescribed by a dermatologist.
• Approvals will be for a maximum of 600 mg at week 0, then 300 mg every two weeks thereafter.
• Initial approval period: 6 months.
• Renewal approval period: 1 year.
DUPIXENT® coverage criteria under the Non-Insured Health Benefits First Nations and Inuit Health Branch (limited use benefit)24
For approval, authorized prescribers will be required to fill out a limited use drugs request form.
Coverage Criteria/Therapeutic Notes
Limited use benefit (prior approval required).
Initial coverage criteria (4 months):
For patients aged 12 years and older with chronic moderate to severe atopic dermatitis who meet all the following criteria:
• Patient has a score greater than or equal to 16 on the Eczema Area and Severity Index (EASI); and
• Patient has a score greater than or equal to 8 on the Dermatology Life Quality Index (DLQI) or Children’s Dermatology Life Quality Index (CDLQI); and
• Body surface area (BSA) of 10% or more is affected; and
• The disease is insufficiently controlled despite the use of topical treatments including at least two medium or high-potency topical corticosteroids and one topical calcineurin inhibitor; and
• intolerance or lack of response to phototherapy or inability to access phototherapy.
Criteria for renewal or for initial coverage in patients currently maintained on DUPIXENT® (12 months):
• Patient has an improvement of at least 75% in the EASI score compared to the baseline level; or
• Patient has an improvement of at least 50% in the EASI score; and
• Patient has had a decrease of at least five points on the DLQI or CDLQI questionnaire compared to the baseline.
Authorizations for dupilumab are given for an initial dose of up to 600 mg followed by a dose of up to 300 mg every two weeks.
DUPIXENT® coverage criteria on PEI Pharmacare formulary through the High Cost Drug Program or the Catastrophic Drug Program26
For approval, authorized prescribers will be required to fill out a request form.
For the treatment of moderate to severe atopic dermatitis in patients 12 years and older who meet all of the following criteria:
• Refractory or have contraindications to an adequate trial of topical prescription therapies
• Refractory, intolerant or have contraindications to an adequate trial of phototherapy (where available), methotrexate, and cyclosporine.
• Baseline Eczema Area and Severity Index (EASI) score of ≥ 7.1 and Physician Global Assessment score of ≥3 at the time of initial request for reimbursement.
• The maximum duration of initial authorization is 6 months.
Renewal criteria:
• Renewal requests must provide proof of beneficial clinical effect, defined as a 75% or greater improvement from baseline in the EASI score (EASI‐75) six months after treatment initiation.
• Proof of maintenance of EASI‐75 response from baseline must be provided for subsequent authorizations.
Clinical note:
• The patient must be under the care of a dermatologist.
• Dupilumab is not to be used in combination with phototherapy or immunosuppressant drugs, such as methotrexate or cyclosporine.
• Approvals will be for a maximum of 600 mg at week 0, then 300 mg every two weeks thereafter
Program eligibility: High Cost Drug Program
DUPIXENT® coverage criteria on Nova Scotia Formulary (Exception Status Drug)25
For approval, authorized prescribers will be required to fill out an Exception Status Drug request form.
For the treatment of moderate to severe atopic dermatitis in patients 12 years of age and older who meet all of the following criteria:
• Refractory or have contraindications to an adequate trial of topical prescription therapies.
• Refractory, intolerant or have contraindications to an adequate trial of phototherapy (where available), methotrexate, and cyclosporine.
• Baseline Physician Global Assessment score of 3 or greater and Eczema Area and Severity Score of 7.1 or greater.
Renewal criteria:
• Requests for renewal must provide proof of beneficial clinical effect defined as a 75% or greater improvement from baseline in the Eczema Area and Severity Index (EASI-75) score six months after treatment initiation.
• Proof of maintenance of EASI-75 response from baseline must be provided for subsequent authorizations.
Clinical note:
• Not to be used in combination with phototherapy or immunosuppressant drugs (e.g., methotrexate, cyclosporine).
Claim notes:
• The patient must be under the care of a dermatologist or allergist.
• Approvals will be for a maximum of 600 mg at week 0, then 300 mg every two weeks thereafter.
• Initial approval period: 6 months.
• Renewal approval period: 1 year.
DUPIXENT® coverage criteria in Northwest Territories under the Extended Health Benefits for Specified Disease Conditions Program (limited use benefit, criteria matches NIHB)24,27
For approval, authorized prescribers will be required to fill out an Extended Health Benefits program request form.
Criteria matches NIHB
NIHB Coverage Criteria/Therapeutic Notes
Limited use benefit (prior approval required).
Initial coverage criteria (4 months):
For patients aged 12 years and older with chronic moderate-to-severe atopic dermatitis who meet all the following criteria:
• Patient has a score greater than or equal to 16 on the Eczema Area and Severity Index (EASI); and
• Patient has a score greater than or equal to 8 on the Dermatology Life Quality Index (DLQI) or Children’s Dermatology Life Quality Index (CDLQI); and
• Body surface area (BSA) of 10% or more is affected; and
• The disease is insufficiently controlled despite the use of topical treatments including at least two medium or high-potency topical corticosteroids and one topical calcineurin inhibitor; and
• Intolerance or lack of response to phototherapy or inability to access phototherapy.
Criteria for renewal or for initial coverage in patients currently maintained on DUPIXENT® (12 months):
• Patient has an improvement of at least 75% in the EASI score compared to the baseline level; or
• Patient has an improvement of at least 50% in the EASI score; and
• Patient has had a decrease of at least five points on the DLQI or CDLQI questionnaire compared to the baseline.
Authorizations for dupilumab are given for an initial dose of up to 600 mg followed by a dose of up to 300 mg every two weeks.
DUPIXENT® coverage criteria on Newfoundland and Labrador Prescription Drug Program (special authorization)28
To request coverage through special authorization, authorized prescribers will be required to fill out a form.
For the treatment of moderate to severe atopic dermatitis in patients 12 years of age and older
who meet all of the following criteria:
Initiation criteria
• Refractory to or have contraindication to an adequate trial with topical prescription therapies, and
• Refractory, intolerant to or have contraindications to phototherapy (where available),
methotrexate, and cyclosporine, and
• Baseline Physician Global Assessment score of 3 or greater and Eczema Area and Severity Score of 7.1.
Renewal criteria
• Requests for renewal must provide proof of beneficial clinical effect defined as a 75% or greater improvement from baseline in the EASI score (EASI-75) six months after treatment initiation.
• Proof of maintenance of EASI-75 response from baseline every six months for subsequent authorizations.
Clinical note
• Dupilumab is not to be used in combination with phototherapy or immunosuppressant drugs, such as methotrexate or cyclosporine.
Claim notes:
• Must be prescribed by a dermatologist.
• Approvals will be for a maximum of 600 mg at week 0, then 300 mg every two weeks thereafter.
• Initial approval period: 6 months.
• Renewal approval period: 6 months.
DUPIXENT®, Sanofi and Freedom logos are trademarks of Sanofi, used under license by sanofi-aventis Canada Inc.
REGENERON® is a trademark of Regeneron Pharmaceuticals, Inc. All rights reserved.
© 2023 sanofi-aventis Canada Inc. All rights reserved.
MAT-CA-2300298
Last updated: 06/2023