Questions about DUPIXENT®
Have a question about DUPIXENT®? See below for frequently asked questions regarding DUPIXENT®. If you do not find the answers you're looking for, request a rep or .
DUPIXENT® basics
Itch reduction was evaluated as a key secondary endpoint in 3 randomized, double-blind, placebo-controlled trials that enrolled 2,119 subjects, 18 years of age and older with moderate-to-severe atopic dermatitis not adequately controlled by topical medication(s).1 Click here for additional clinical trial design information.
Itch reduction with DUPIXENT® was rapid and sustained.
Significant pruritus relief with DUPIXENT® vs. placebo was seen as early as Week 2 (p<0.01) in both pivotal monotherapy trials with a significant reduction in itch at Week 16.1,3
• 41% of patients achieved a ≥4-point improvement in the Pruritus Numerical Rating Scale (NRS) with DUPIXENT® at Week 16 in SOLO 1 vs. 12% with placebo1
• 36% of patients achieved a ≥4-point improvement in the Pruritus NRS with DUPIXENT® at Week 16 in SOLO 2 vs. 10% with placebo1
• 9.4% of patients achieved a ≥4-point improvement in the Pruritus NRS with DUPIXENT® at Week 2 in SOLO 1 vs. 3.3% with placebo1
• 10.7% of patients achieved a ≥4-point improvement in the Pruritus NRS with DUPIXENT® at Week 2 in SOLO 2 vs. 0.9% with placebo1
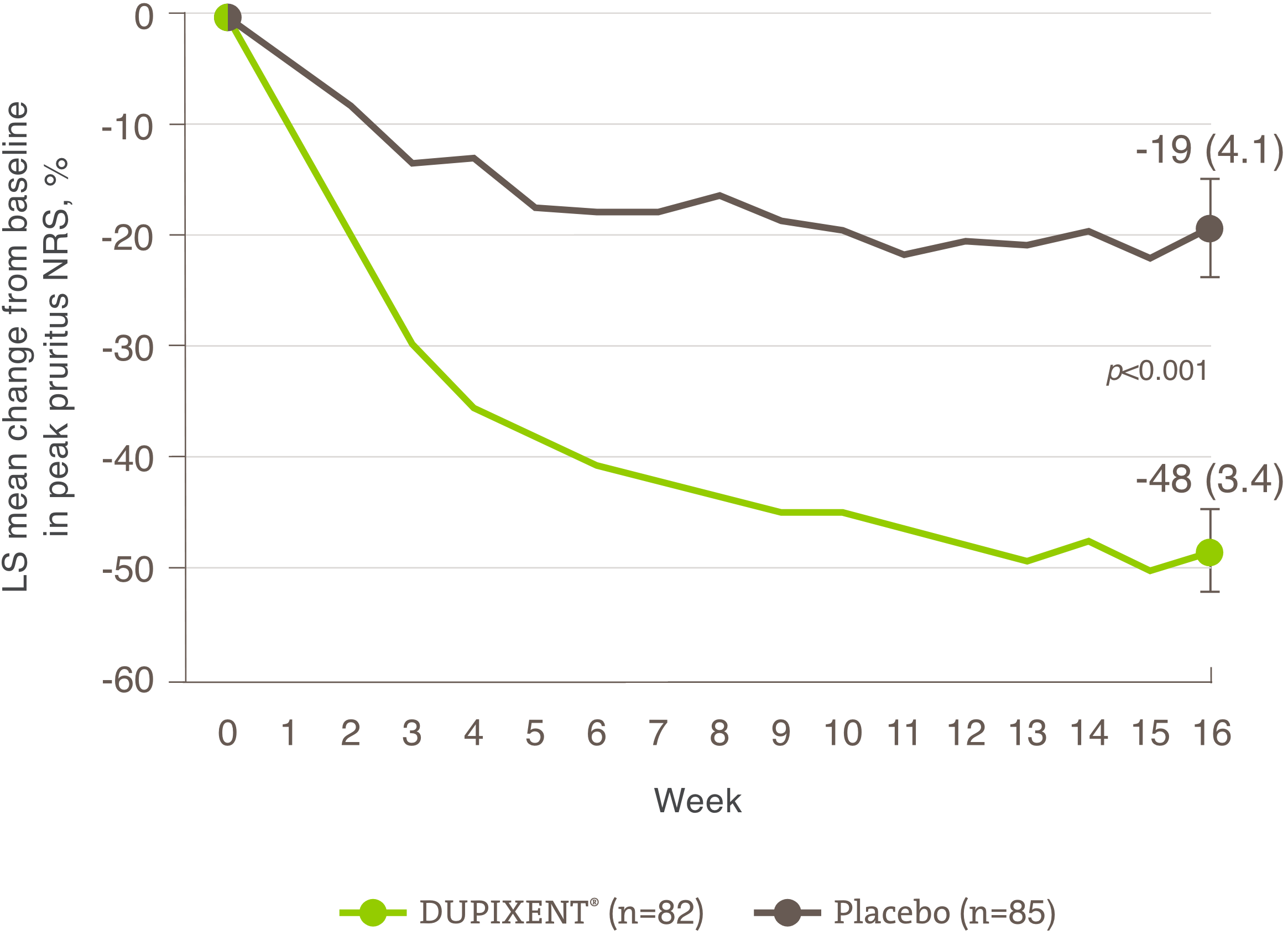
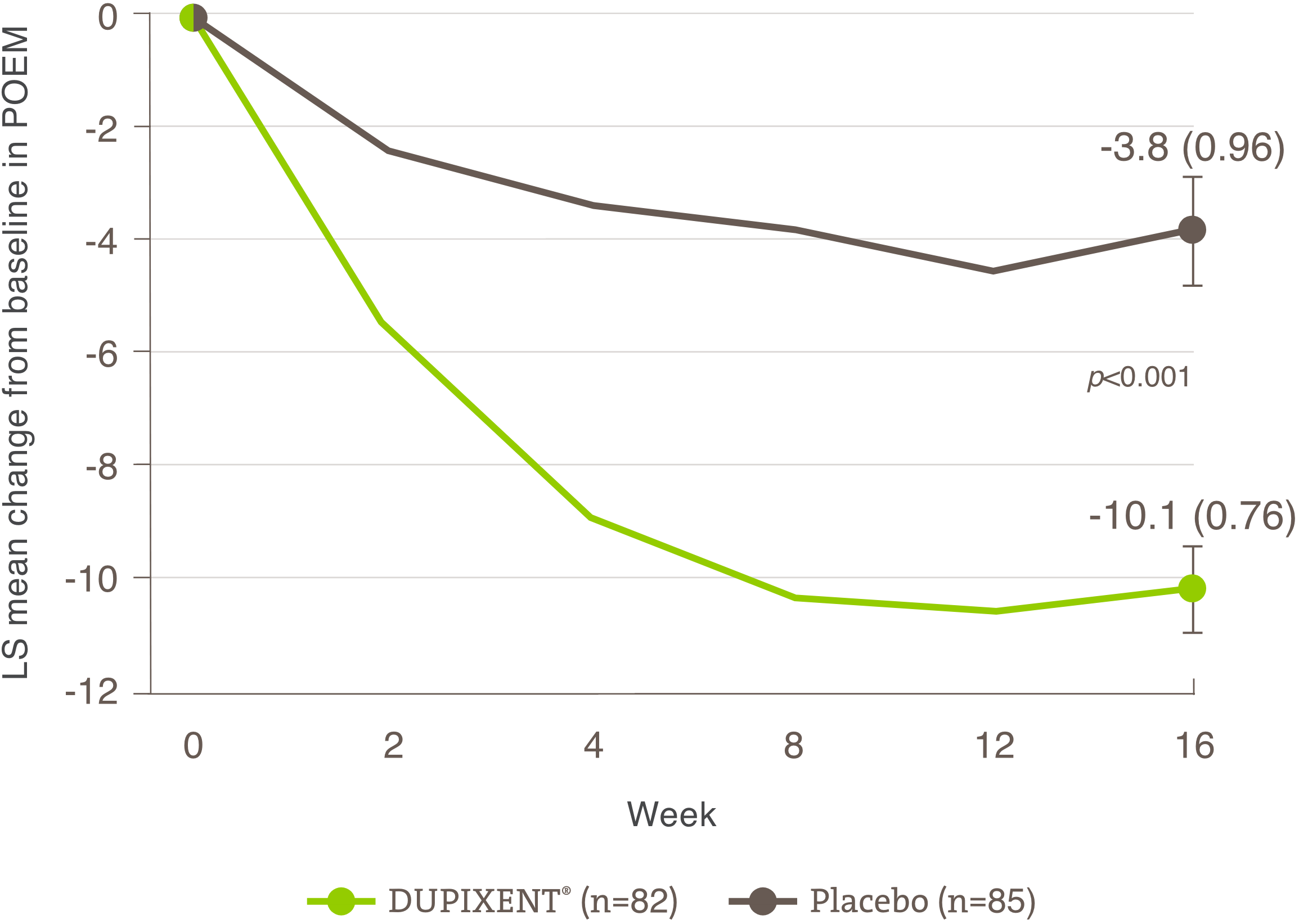
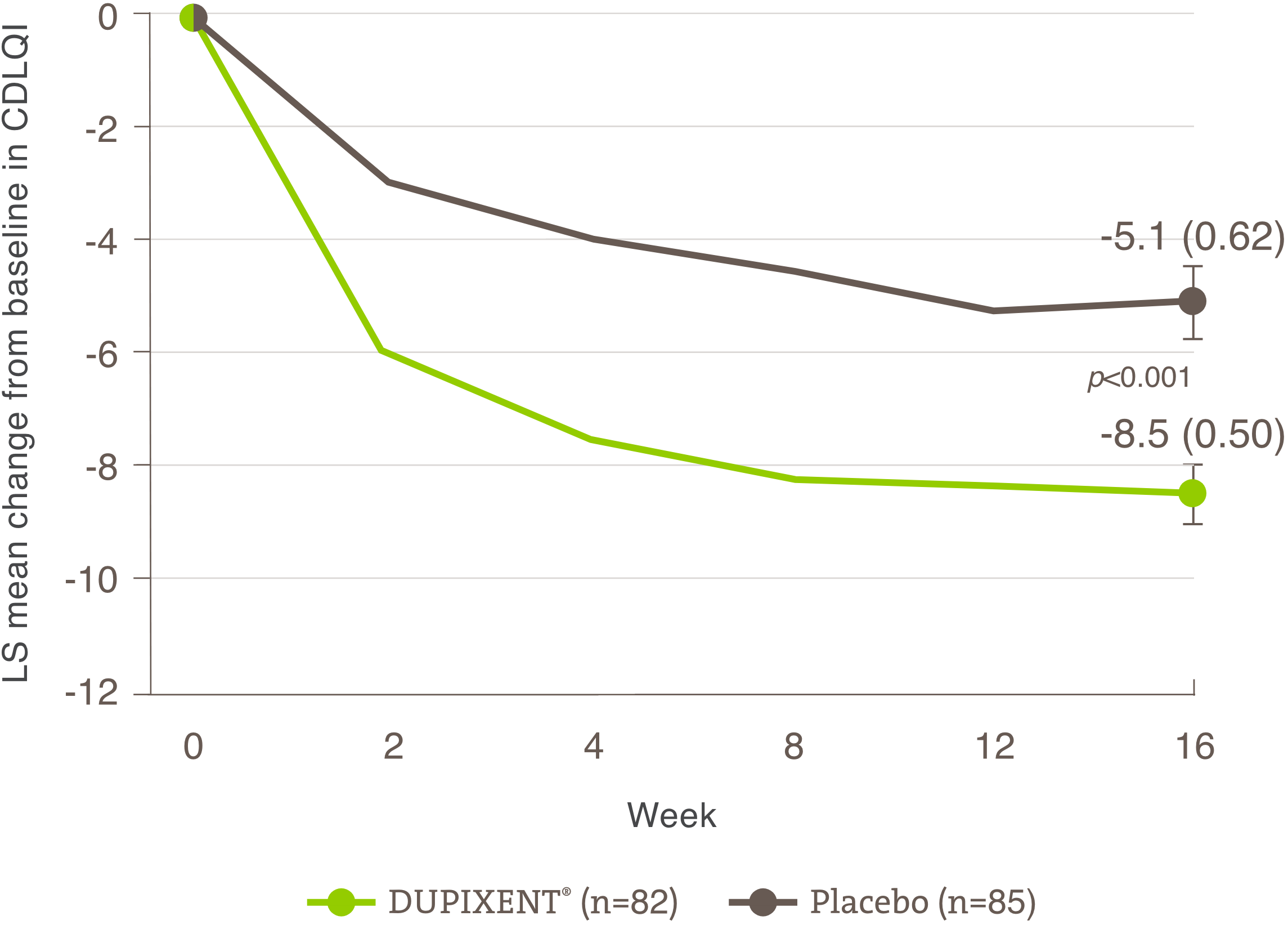
Itch reduction was evaluated as a key secondary endpoint in a multicenter, randomized, double-blind, placebo-controlled trial investigating the efficacy and safety of DUPIXENT® monotherapy for 16 weeks in 251 adolescent AD patients (12 to 17 years old) with moderate-to-severe AD who were not adequately controlled with topical medication.1 Click here for additional clinical trial design information.
Significant reduction in itch was seen at Week 16.1
• 37% of patients achieved a ≥4-point improvement in the Pruritus Numerical Rating Scale (NRS) with DUPIXENT® at Week 16 vs. 5% with placebo1†
• There was a 48% mean improvement in NRS with DUPIXENT® at Week 16 vs. 19% with placebo1
A significantly greater proportion of subjects randomized to DUPIXENT® achieved a rapid improvement in the pruritus NRS compared to placebo (defined as >4-point improvement as early as Week 4; nominal p<0.001).1
The proportion of subjects responding on the pruritus NRS continued to increase through the treatment period. The improvement in pruritus NRS occurred in conjunction with the improvement of objective signs of atopic dermatitis.1
† Subjects who received rescue treatment or with missing data were considered as non-responders (59% and 21% in the placebo and DUPIXENT® arms, respectively).1
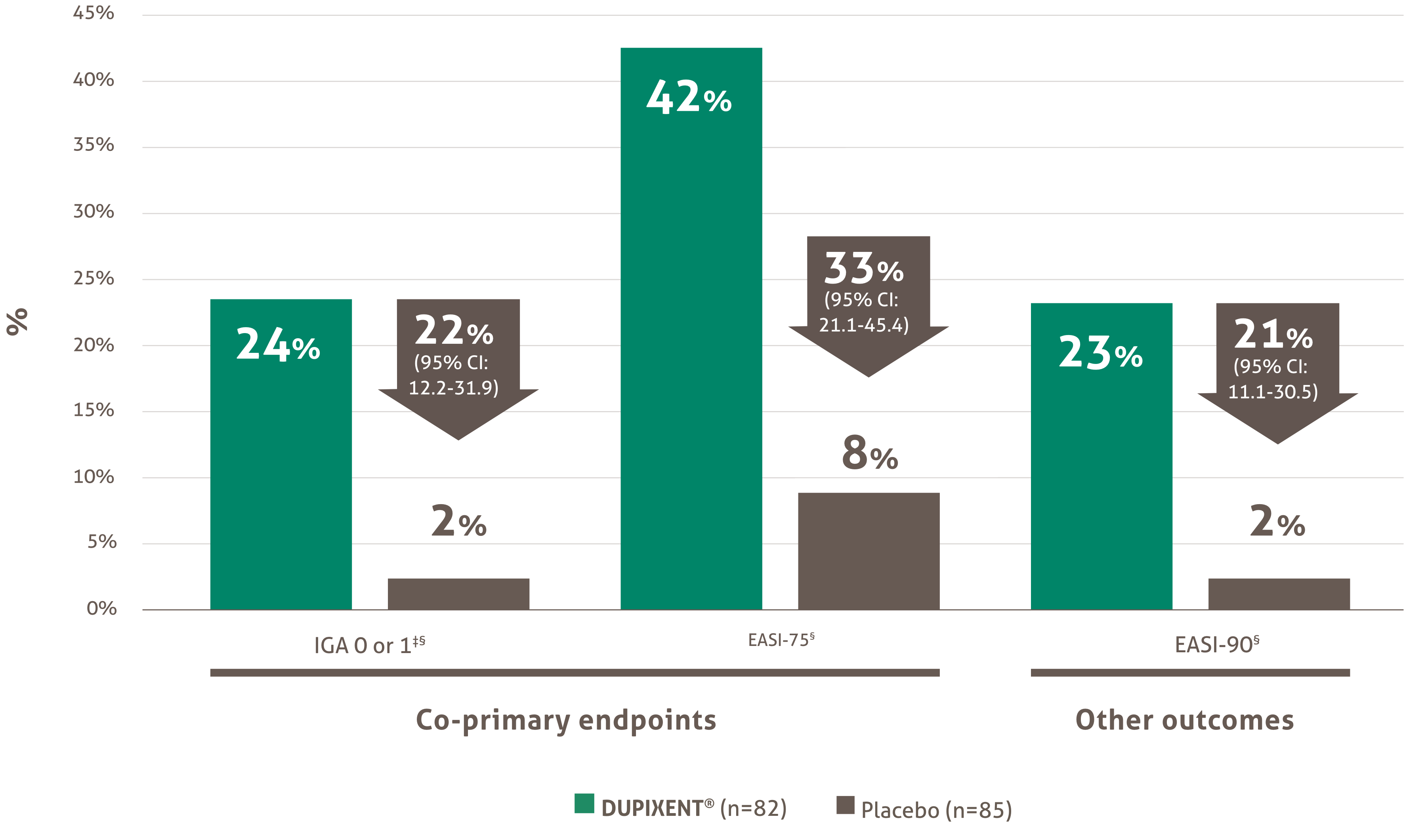
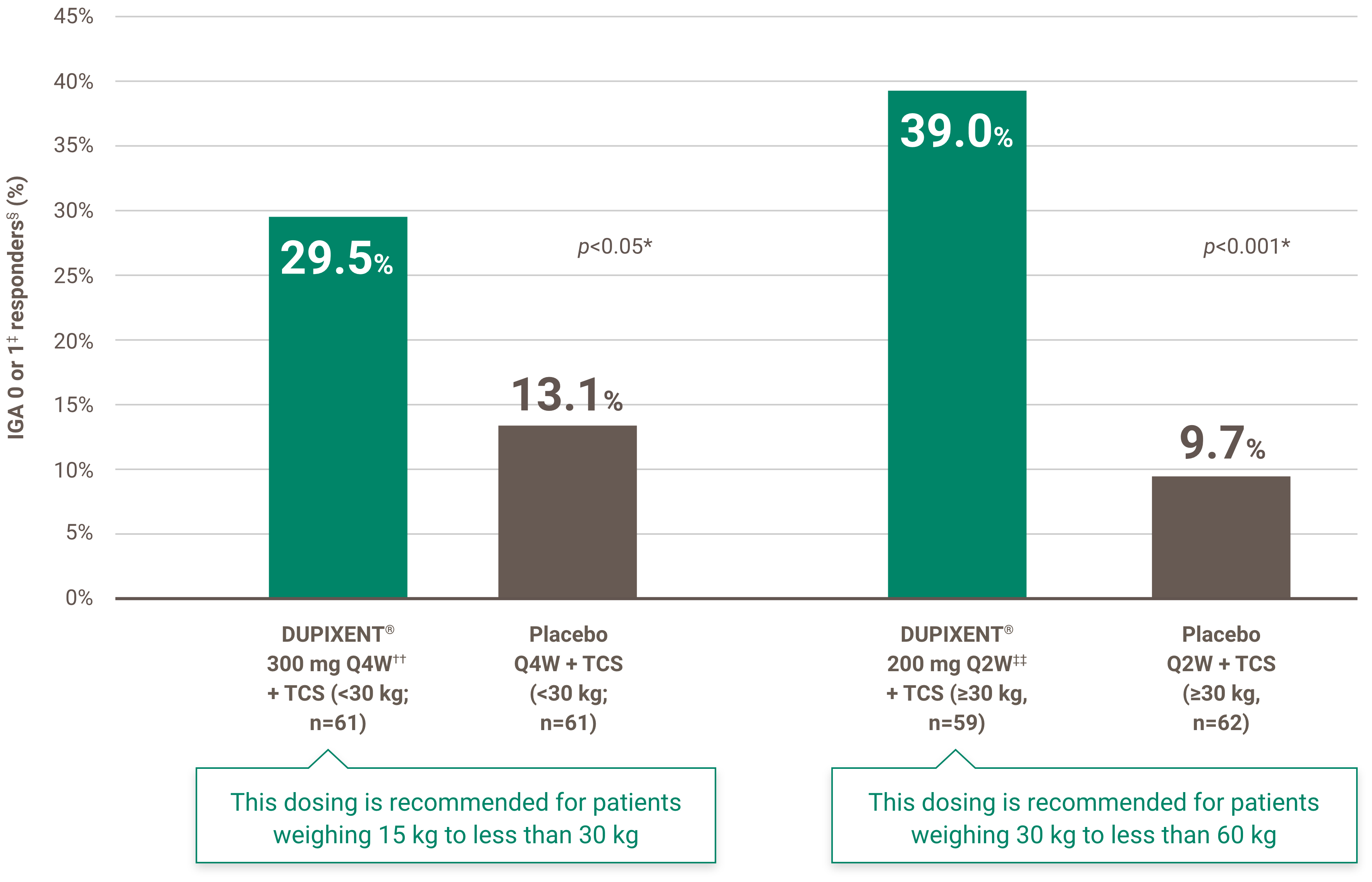
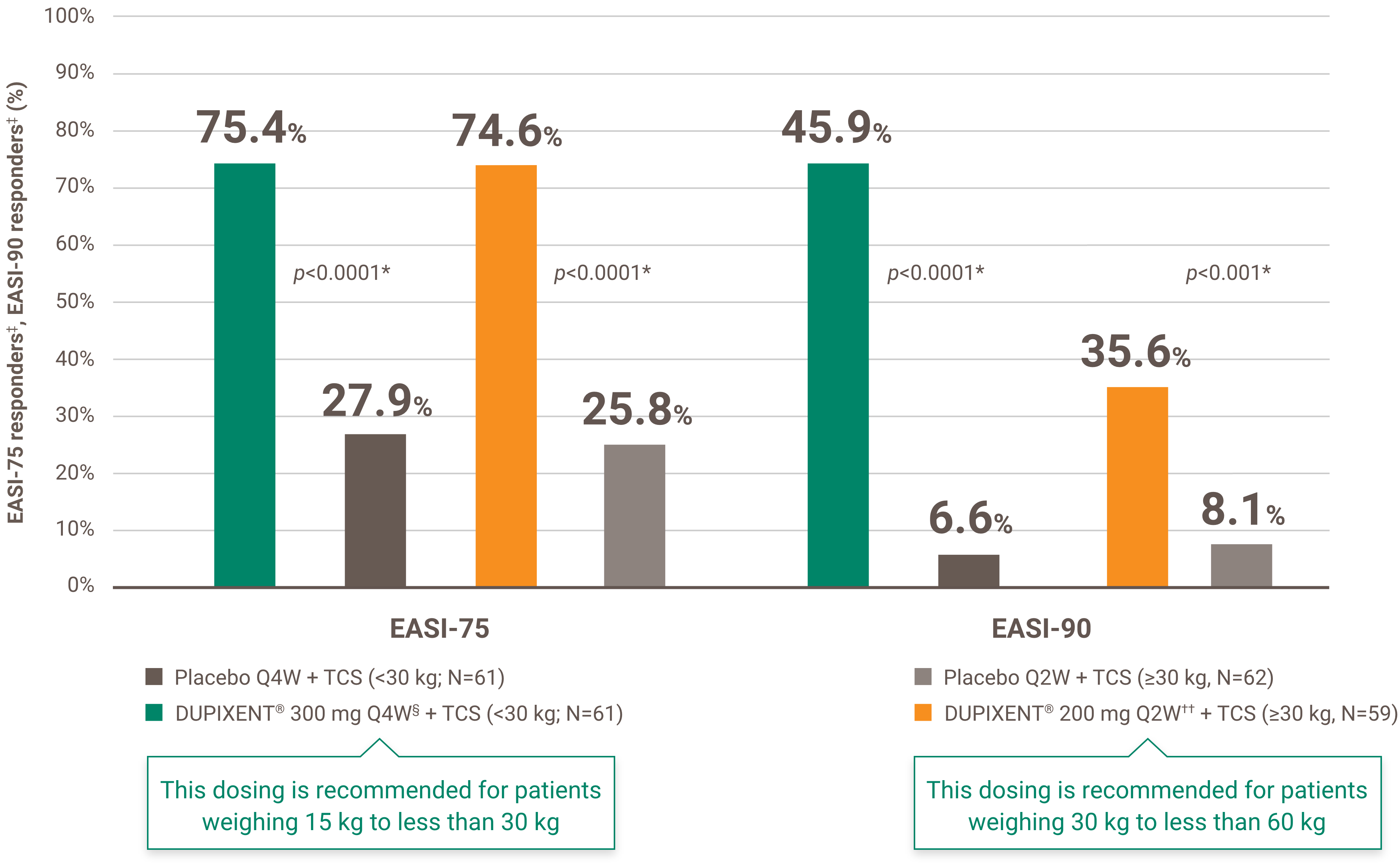
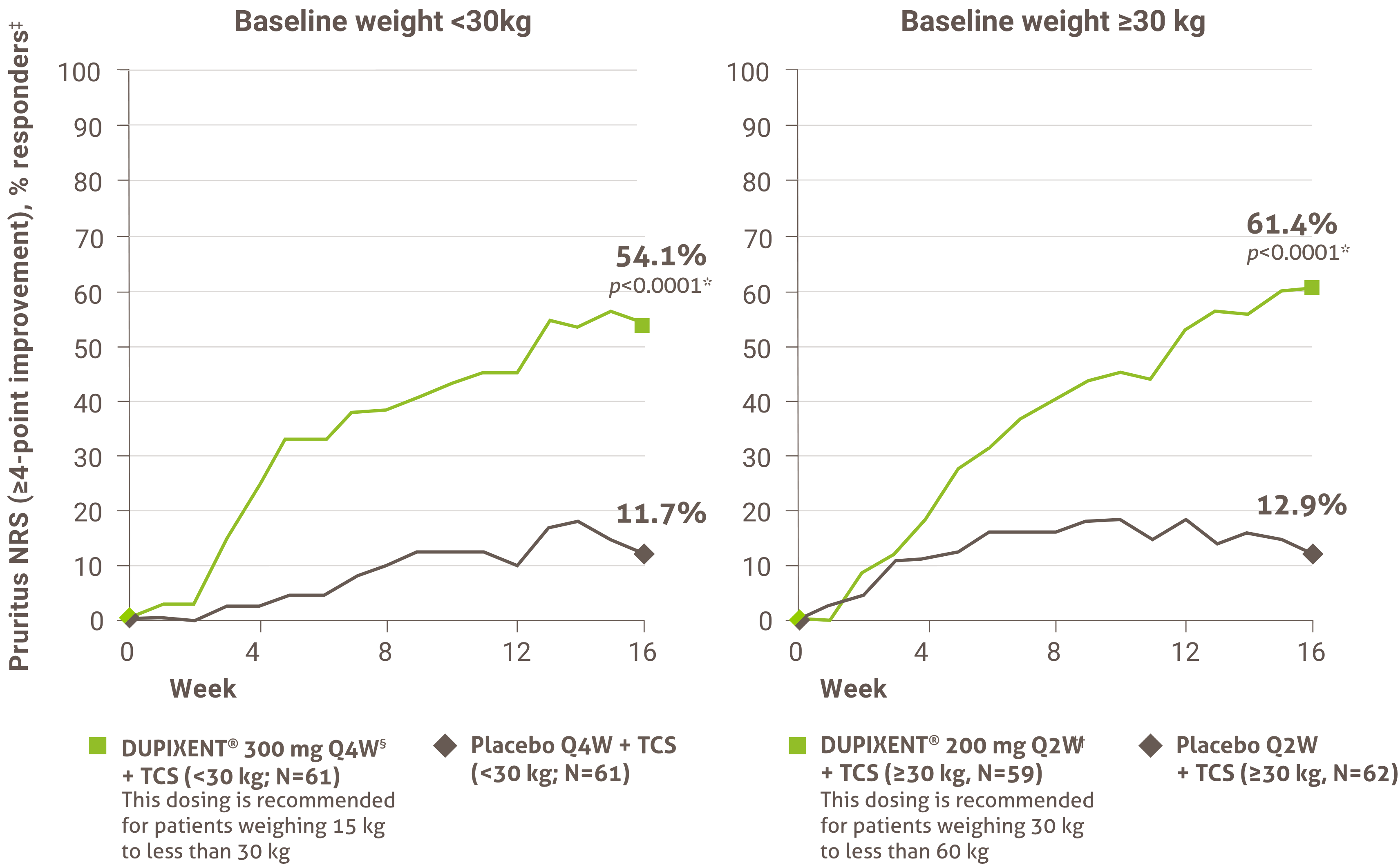
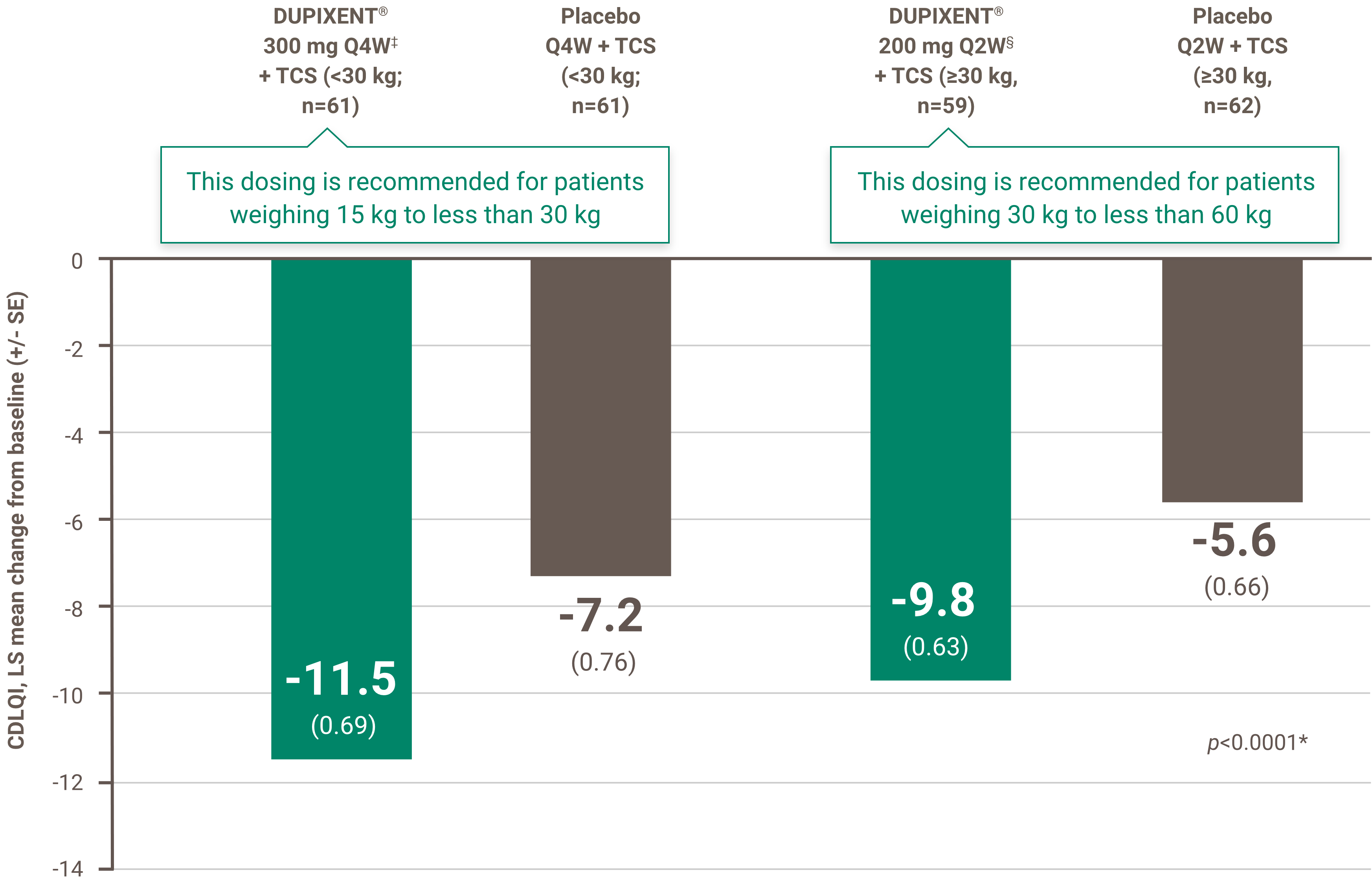
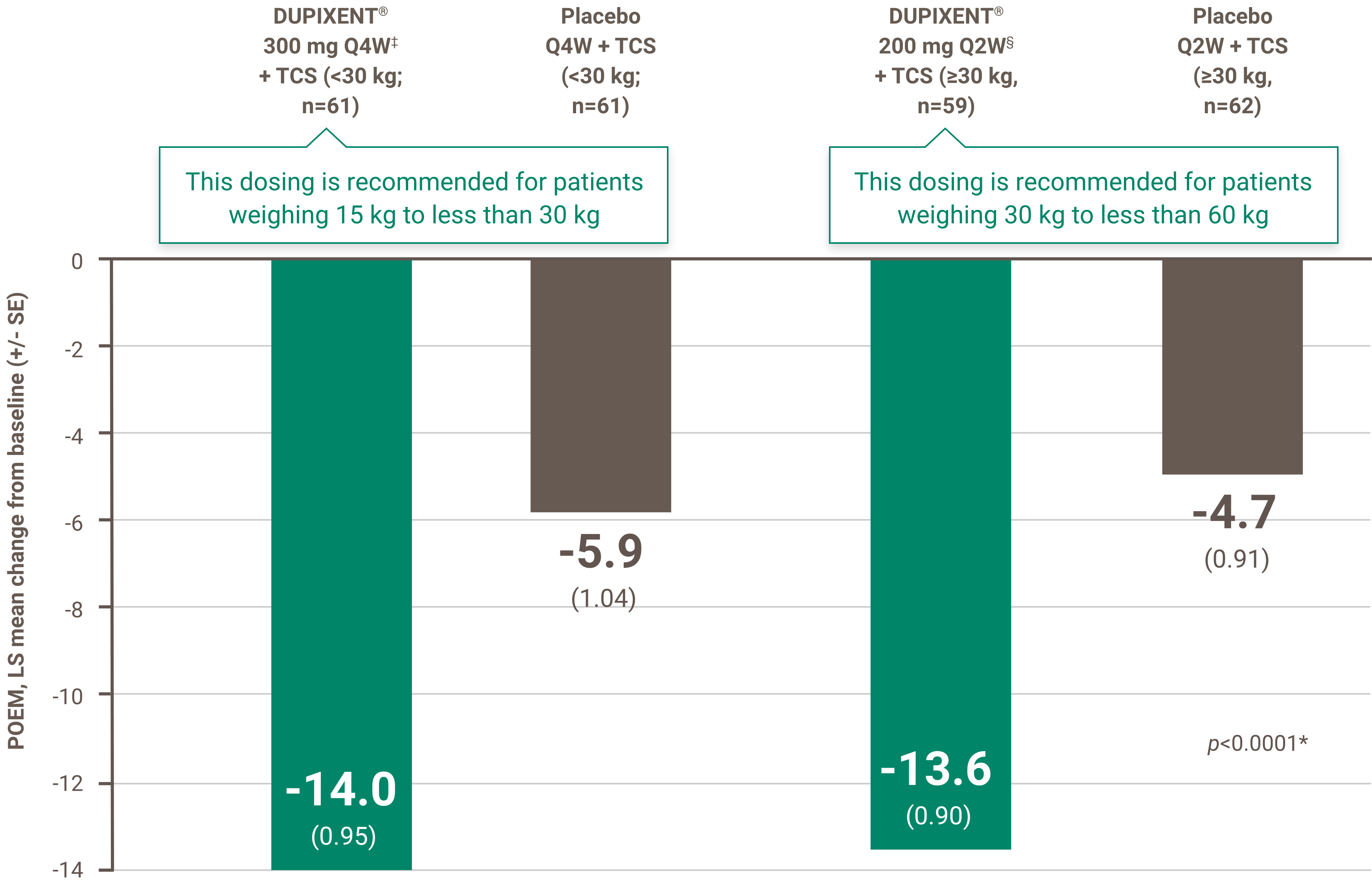
Itch reduction was evaluated as a key secondary endpoint in a multicenter, randomized, double-blind, placebo-controlled trial investigating the efficacy and safety of DUPIXENT® + TCS for 16 weeks in 367 pediatric AD patients (6 to 11 years old) with severe AD who were not adequately controlled with topical medication.1 Click here for additional clinical trial design information.
At Week 16:1,4†
• 54.1% of patients treated with DUPIXENT® 300 mg Q4W + TCS (<30 kg)‡ vs. 11.7% in placebo achieved a ≥4-point improvement in pruritus NRS (nominal p-value <0.05)
• 61.4% of patients treated with DUPIXENT® 200 mg Q2W + TCS (≥30 kg)§ vs. 12.9% in placebo achieved a ≥4-point improvement in pruritus NRS (nominal p-value <0.001)
A greater proportion of subjects randomized to DUPIXENT® + TCS achieved an improvement in the peak pruritus NRS compared to placebo + TCS (defined as ≥4-point improvement at week 4).1
† Subjects who received rescue treatment or who had missing data were classified as non-responders.
‡ Subjects received an initial dose of 600 mg dupilumab. DUPIXENT® 300 mg Q4W + TCS is recommended for patients weighing 15 kg to less than 30 kg.
§ Subjects received an initial dose of 400 mg dupilumab. DUPIXENT® 200 mg Q2W + TCS is recommended for patients weighing 30 kg to less than 60 kg.
Clinical trials
CHRONOS was a 52-week pivotal clinical trial evaluating the efficacy and safety profile of DUPIXENT® in patients with moderate-to-severe atopic dermatitis, inadequately controlled with topical prescription therapies or when those therapies are not advisable. The 421 adult patients in CHRONOS were randomized to DUPIXENT® + concomitant TCS or placebo + TCS.1,5
Disease severity was defined by an IGA score ≥3 in the overall assessment of atopic dermatitis lesions on a severity scale of 0 to 4, an EASI score ≥16 on a scale of 0 to 72, and a minimum body surface area involvement of ≥10%.1
Studies demonstrated significant improvement with DUPIXENT® + TCS in achieving clear (IGA 0) or almost-clear skin (IGA 1) (the primary endpoint at 16 weeks).1,5
• 39% of adult patients treated with DUPIXENT® + TCS achieved clear or almost-clear skin at 16 weeks vs. 12% with placebo + TCS (p<0.0001)1,5
• 36% of adult patients achieved clear or almost-clear skin (IGA 0 or 1) with DUPIXENT® + TCS at 52 weeks vs. 13% with placebo + TCS (p<0.0001)1,5
• 69% of adult patients treated with DUPIXENT® + TCS demonstrated improvement in EASI-75 at 16 weeks vs. 23% with placebo + TCS (p<0.0001)1,5
The most common adverse reactions (incidence ≥1%) observed at Week 16 in adult patients were injection-site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, eosinophilia, and dry eye.1
Additionally, the safety profile of DUPIXENT® + TCS through Week 52 was generally consistent with the safety profile observed at Week 16.1
The safety of DUPIXENT® was assessed in a study of 250 patients 12 to 17 years of age with moderate-to-severe atopic dermatitis. The safety profile of DUPIXENT® in these patients followed through Week 16 was consistent with the safety profile from studies in adults with atopic dermatitis.1
The long-term safety of DUPIXENT® was assessed in an open-label extension study in patients 12 to 17 years of age with moderate-to-severe atopic dermatitis (AD-1434). The safety profile of DUPIXENT® in patients followed through Week 52 was consistent with the safety profile observed at Week 16 in AD-1526 study. Overall, the safety profile of DUPIXENT® observed in adolescents was consistent with that seen in adults with atopic dermatitis.1
The safety of DUPIXENT® was assessed in a trial of 367 patients 6 to 11 years of age with severe atopic dermatitis (AD-1652). The safety profile of DUPIXENT® + TCS in these patients through Week 16 was consistent with the safety profile established in adults and adolescents with atopic dermatitis.1
The longer-term safety of DUPIXENT® + TCS was assessed in a 52-week open-label extension study including 368 subjects 6 to 11 years of age with atopic dermatitis (AD-1434) who had participated in a prior atopic dermatitis study of DUPIXENT®. The safety profile of DUPIXENT® + TCS in subjects followed through Week 52 from trial AD-1434 was consistent with that observed at Week 16 from trial AD-1652.1
Overall, the safety profile of DUPIXENT® + TCS observed in children was consistent with that seen in adults and adolescents with atopic dermatitis.1
Using DUPIXENT®
The recommended dose of DUPIXENT® in adults is an initial dose of two injections (600 mg), and then one injection (300 mg) every other week.
The recommended dose in adolescents and pediatrics (6 to 17 years of age) is based on body weight:

DUPIXENT® can be used with or without TCS.1
The 52-week adult study was designed to evaluate therapy with DUPIXENT® in combination with TCS at Week 16 and long-term efficacy and safety profile at Week 52.1,5
The safety profile of DUPIXENT® + TCS through Week 52 is generally consistent with the safety profile observed at Week 16.1
The most common adverse reactions (incidence ≥1%) observed at Week 16 were injection-site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, eosinophilia, and dry eye.1
Yes, DUPIXENT® can be used concomitantly with topical calcineurin inhibitors (TCIs), but, as stated in the DUPIXENT® label, they should be reserved for problem areas only, such as the face, neck, and intertriginous and genital areas.1
In the adult 52-week CHRONOS trial, patients were permitted to use TCIs as needed for problem areas only.1
For more details about DUPIXENT® concomitant usage and dosing, please visit the Dosing and Administration section of this website.
![]()
Dosing and Administration
Thinking about prescribing DUPIXENT®? Find helpful information to get started.
![]()
Connect With a Rep
Have questions about DUPIXENT®? Get answers from a representative.
DUPIXENT®, Sanofi and Freedom logos are trademarks of Sanofi, used under license by sanofi-aventis Canada Inc.
REGENERON® is a trademark of Regeneron Pharmaceuticals, Inc. All rights reserved.
© 2023 sanofi-aventis Canada Inc. All rights reserved.
MAT-CA-2300298
Last updated: 06/2023