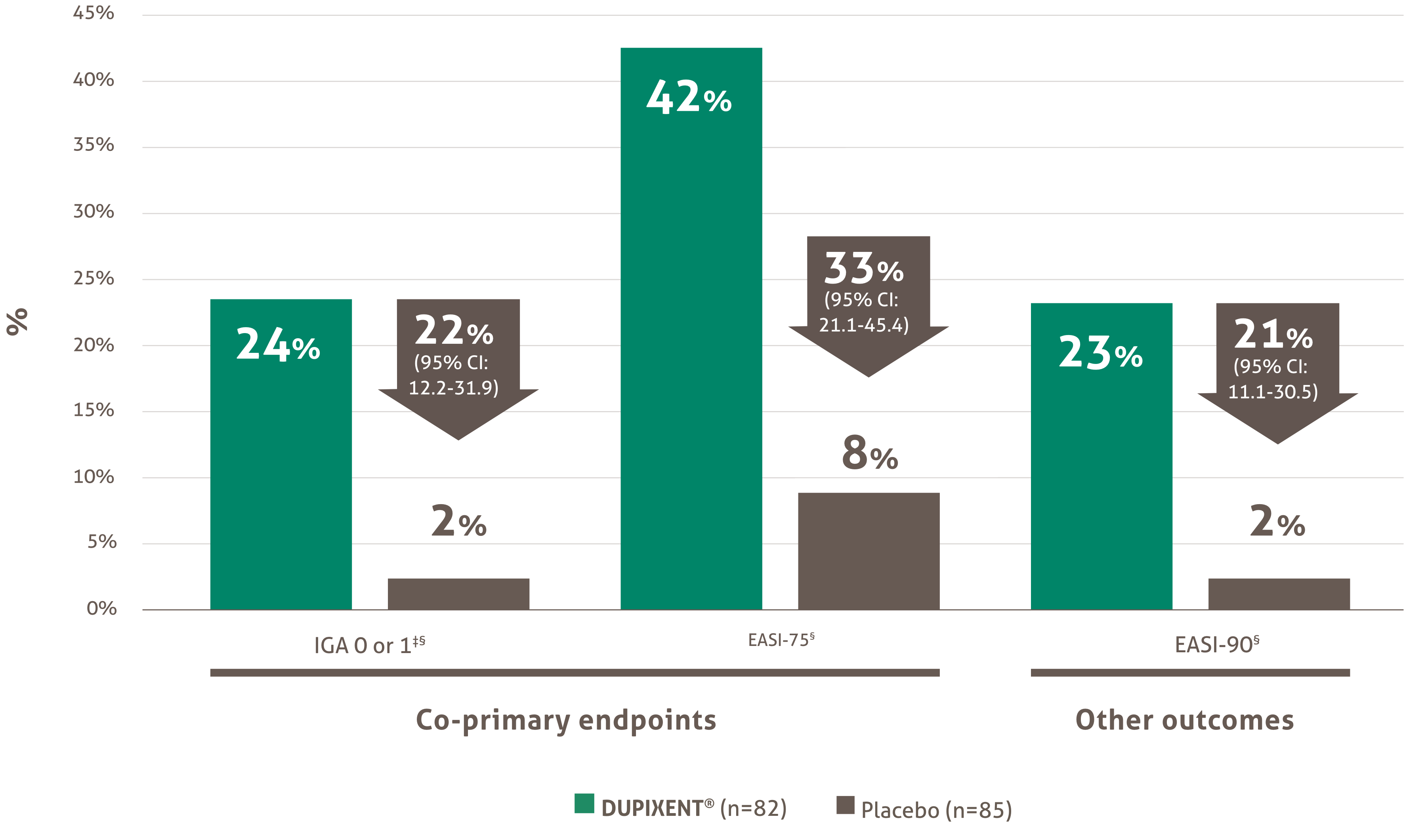
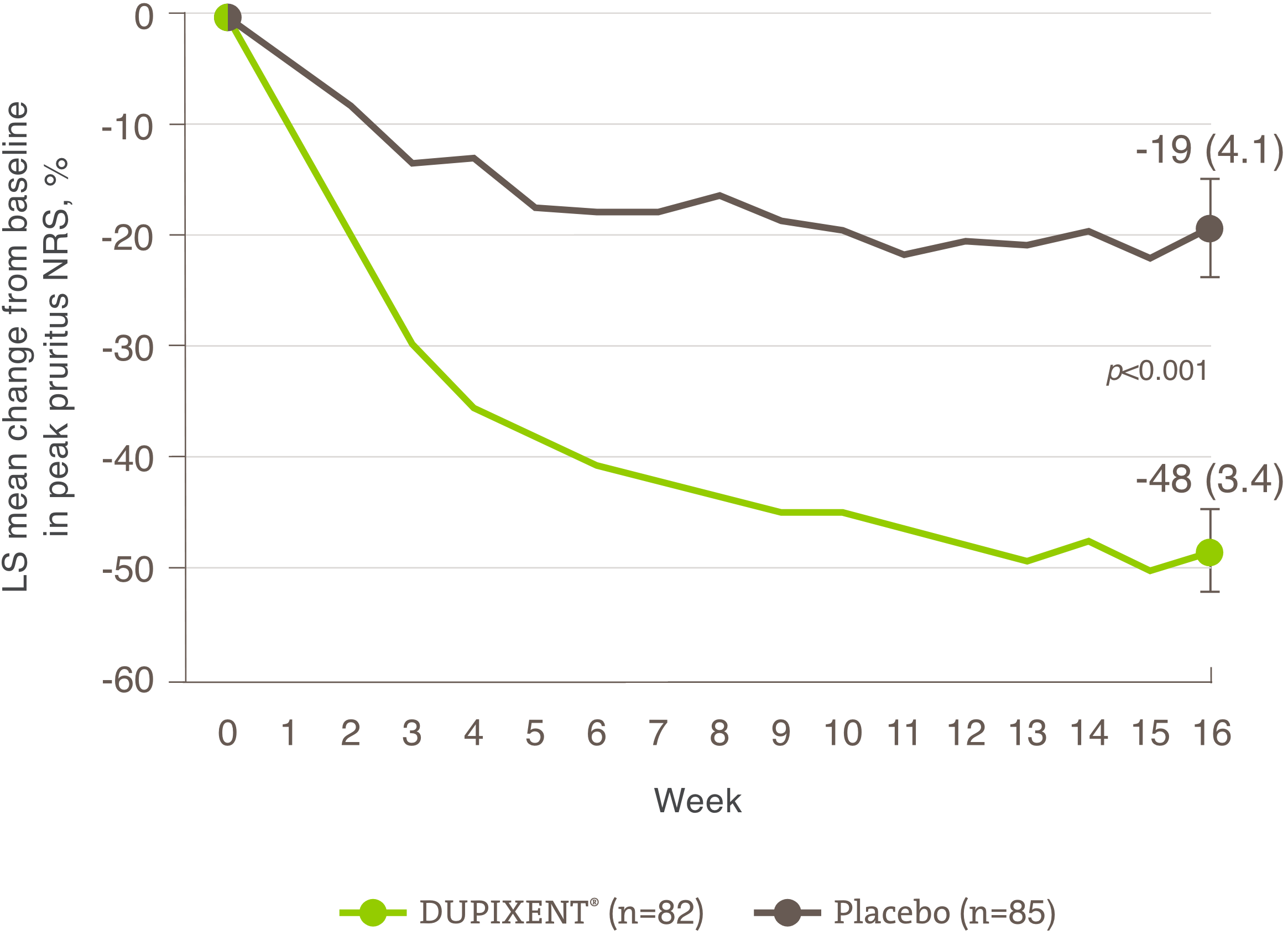
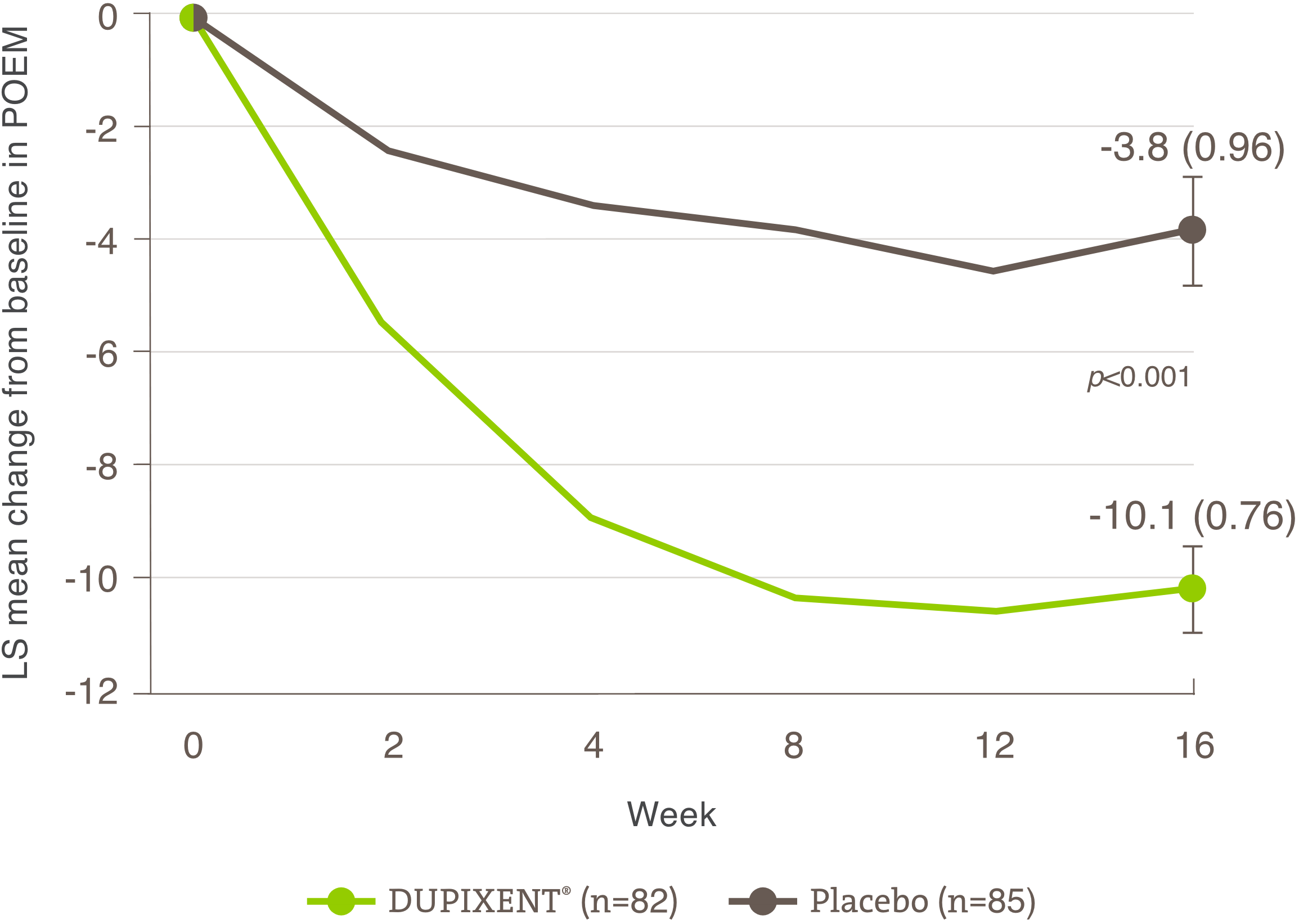
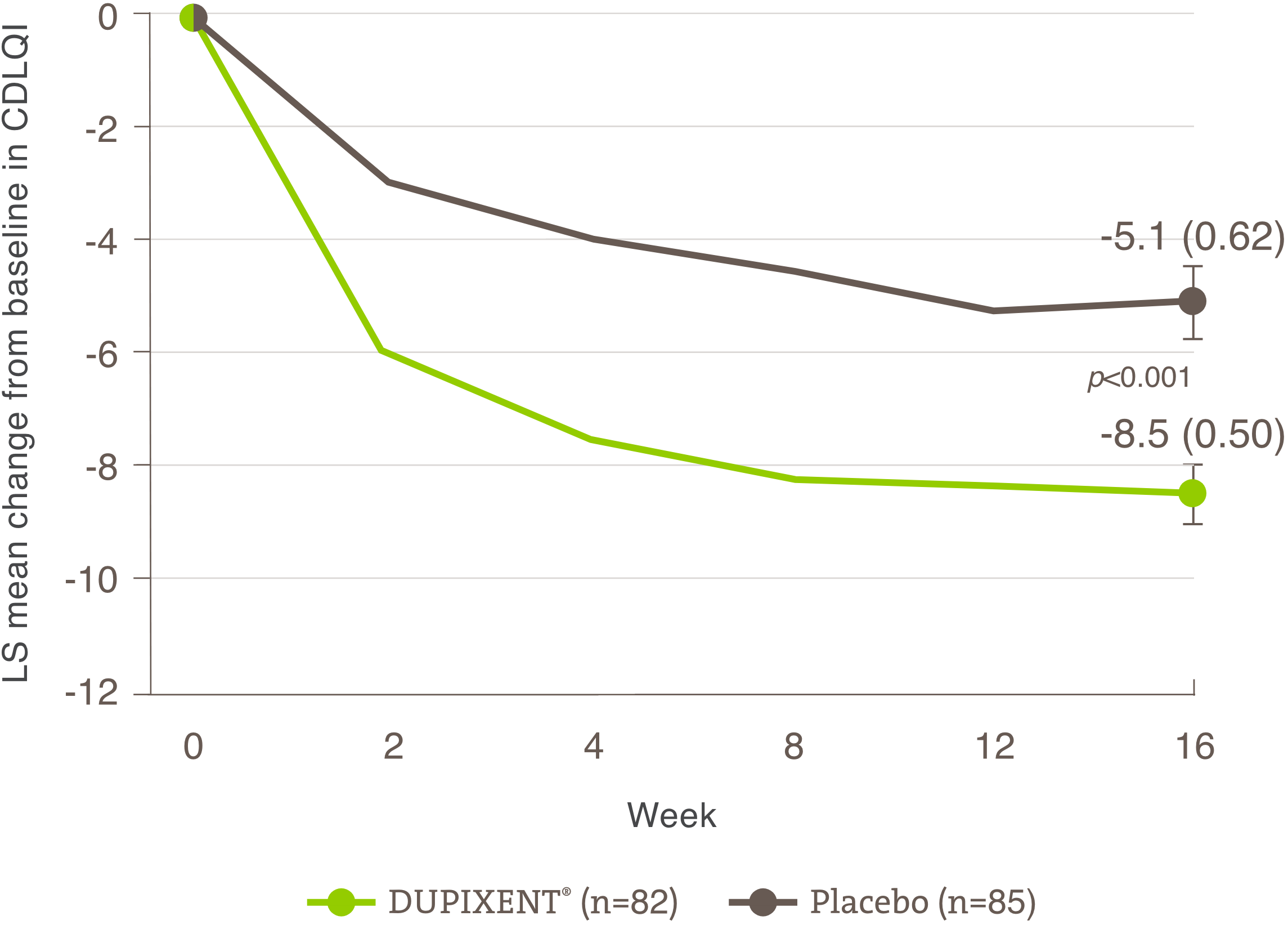
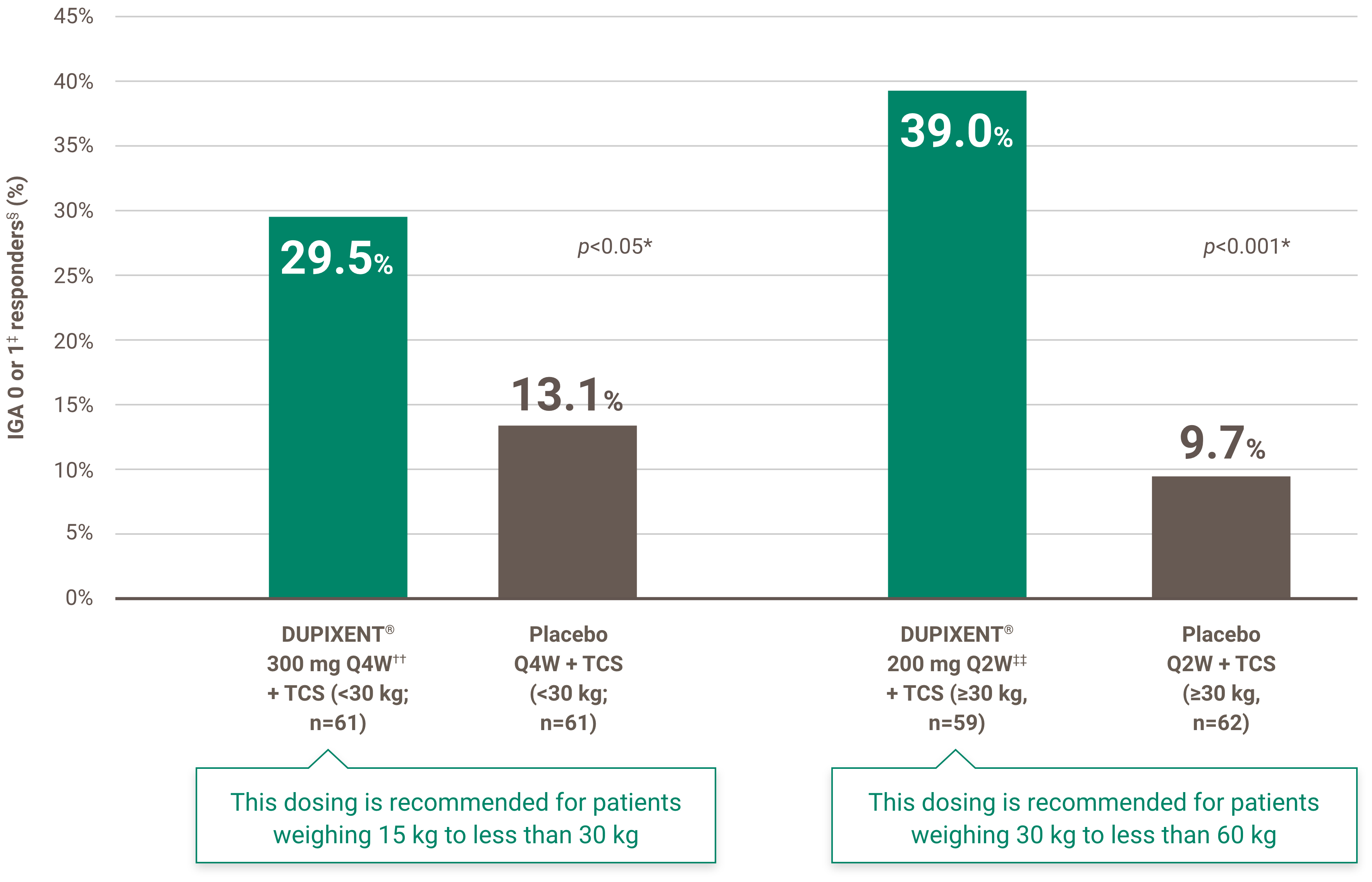
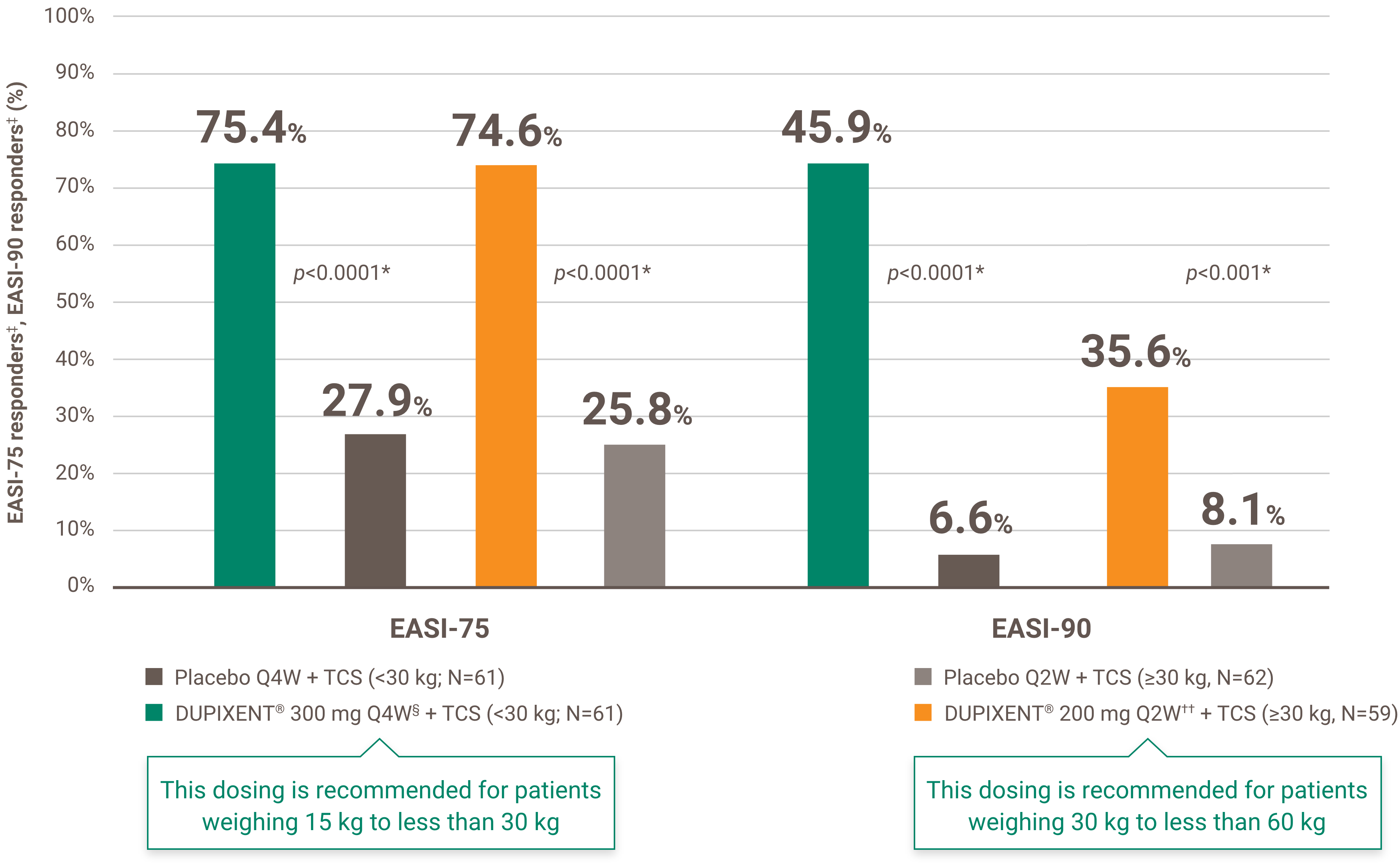
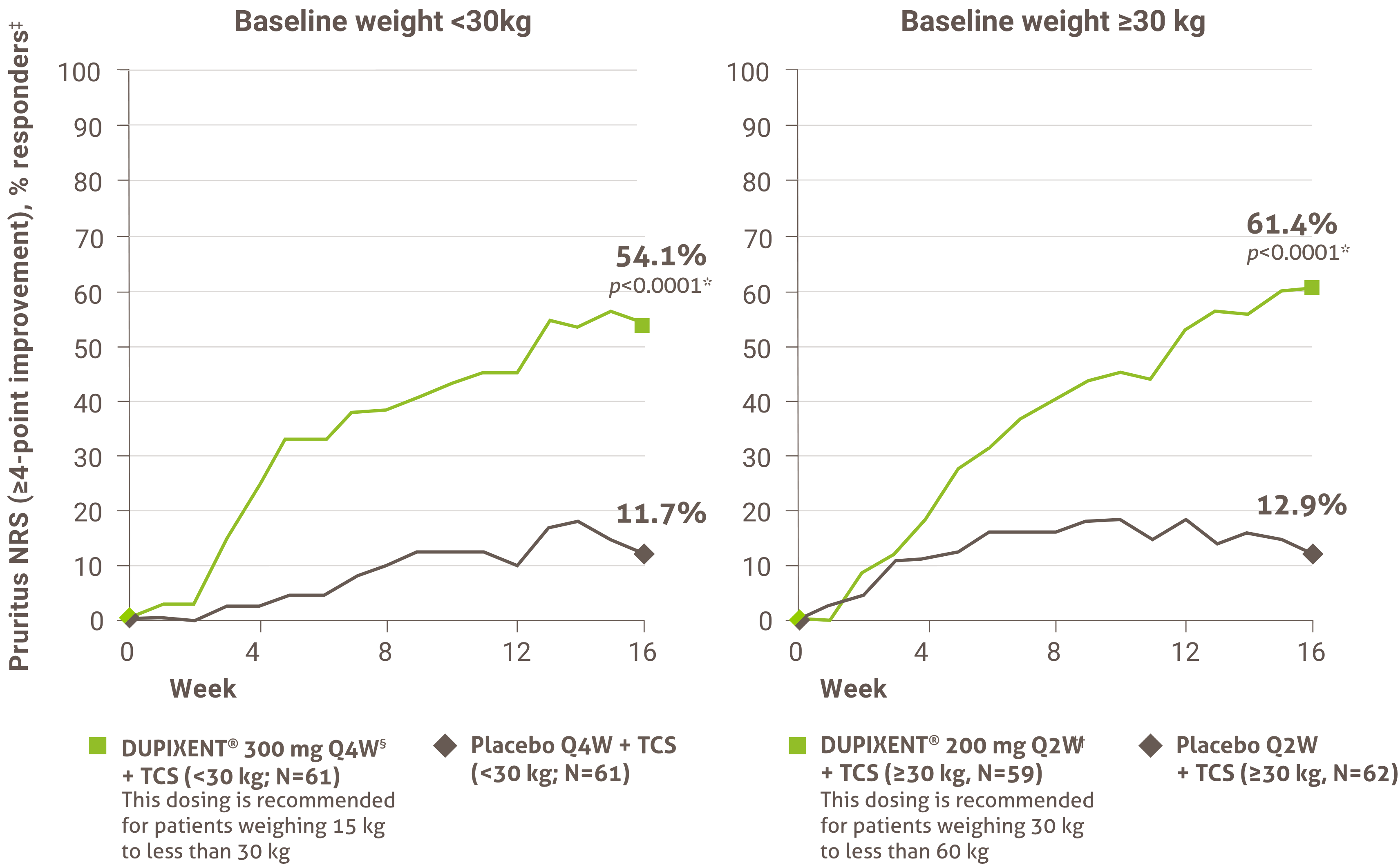
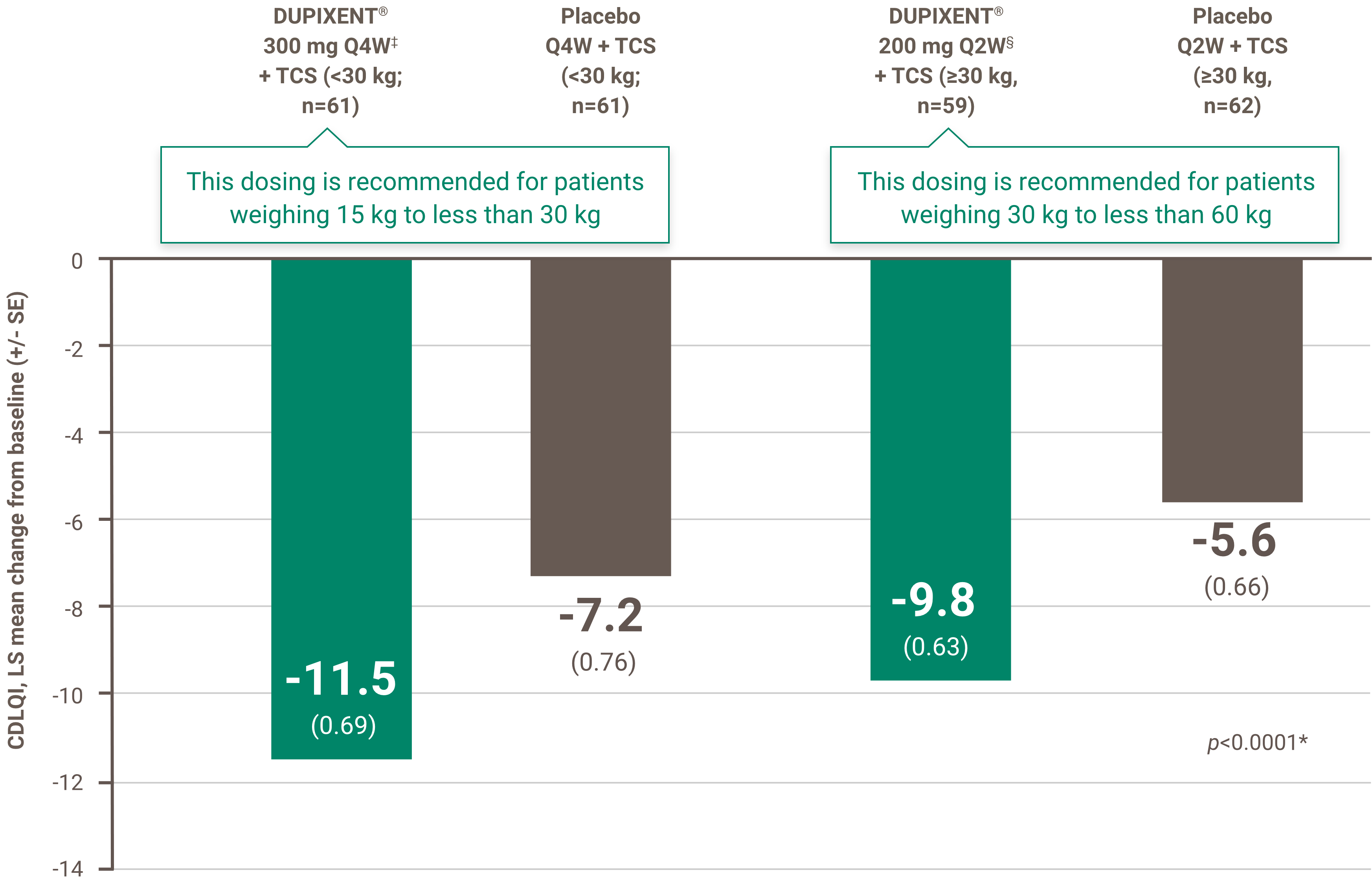
Indication and clinical use:
Atopic Dermatitis
DUPIXENT® (dupilumab injection) is indicated for the treatment of patients aged 6 years and older with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
DUPIXENT® can be used with or without topical corticosteroids.
• No dose adjustment is recommended for elderly patients
• Efficacy and safety of DUPIXENT® in pediatric patients with atopic dermatitis below the age of 6 years have not been established
Asthma
DUPIXENT® (dupilumab injection) is indicated as an add-on maintenance treatment in patients aged 6 years and older with severe asthma with a type 2/eosinophilic phenotype or oral corticosteroid-dependent asthma.
DUPIXENT® is not indicated for relief of acute bronchospasm or status asthmaticus.
• Efficacy and safety in pediatric patients with asthma below the age of 6 years have not been established
Chronic Rhinosinusitis with Nasal Polyposis
DUPIXENT® (dupilumab injection) is indicated as an add-on maintenance treatment with intranasal corticosteroids in adult patients with severe chronic rhinosinusitis with nasal polyposis (CRSwNP) inadequately controlled by systemic corticosteroids and/or surgery
• No dose adjustment is recommended for elderly patients.
• Efficacy and safety of DUPIXENT® in pediatric patients with CRSwNP have not been established
Contraindications:
Hypersensitivity to DUPIXENT® or to any ingredient in the formulation or component of the container.
Warnings and precautions:
• Reduction of corticosteroid dosage
• Systemic hypersensitivity reactions
• Elevation of blood eosinophils
• Eosinophilic pneumonia
• Not studied with live vaccines
• Conjunctivitis and keratitis
• Patients with helminth infection
• Patients with concomitant atopic conditions
• Acute asthma symptoms or deteriorating disease
• Pregnant and nursing women
For more information:
Consult the Product Monograph at http://products.sanofi.ca/en/dupixent-en.pdf for important information relating to adverse drug reactions, drug interactions and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling 1-800-589-6215.
References:
1. DUPIXENT® Product Monograph, sanofi-aventis Canada Inc., March 25, 2022.
2. Johnston A, et al. Immunobiologics: Targeted Therapy Against Cytokines, Cytokine Receptors, and Growth Factors in Dermatology. Fitzpatrick's Dermatology, 9e. New York, NY: McGraw-Hill. Available at: http://accessmedicine.mhmedical.com/content.aspx?bookid=2570§ionid=210444001. Accessed August 19, 2019.
3. Simpson EL, et al. NEJM 2016;375:2335-48.
4. Paller et al. J Am Acad Dermatol 2020; https://doi.org/10.1016/j.jaad.2020.06.054.
5. Blauvelt A, et al. Lancet. 2017;389:2287–2303.
6. Data on file. sanofi-aventis Canada Inc.
7. Phan NQ, et al. Acta Derm Venereol 2012;92(5):449-581.
8. Data on file. sanofi-aventis Canada Inc.
9. International Eczema Council. Investigator Global Assessment Scale. Available at: http://www.eczemacouncil.org/research/investigator-global-assessment-scale/. Accessed August 19, 2019.
10. Harmonising Outcome Measures for Eczema (HOME). EASI Scoring System. January 2017. Available at: http://www.homeforeczema.org/documents/easi-user-guide-jan-2017-v3.pdf. Accessed March 4, 2019.
11. Pruritus Resources. Numerical Rating Scale (NRS). Available at: http://www.pruritussymposium.de/numericalratingscale.html. Accessed May 24, 2018.
12. Finlay AY and Khan GK. Clin Exp Dermatol 1994;19(3):210-6.
13. Cardiff University School of Medicine - Department of Dermatology. DLQI Instructions for use and scoring. Available at: http://sites.cardiff.ac.uk/dermatology/quality-of-life/dermatology-quality-of-life-index-dlqi/dlqi-instructions-for-use-and-scoring/ . Accessed May 10, 2018.
14. Charman C, et al. Arch Dermatol 2004 140:1513-19.
15. University of Nottingham – Centre of Evidence Based Dermatology. POEM – Patient Oriented Eczema Measure. Available at: https://www.nottingham.ac.uk/research/groups/cebd/resources/poem.aspx. Accessed May 25, 2018.
16. Simpson EL et al. JAMA Dermatol 2019; doi: 10.1001/jamadermatol.2019.3336.
17. Lewis-Jones MS, Finlay AY. Br J Dermatol 1995;132(6):942-9.
18. Paller et al. J Am Acad Dermatol 2020; https://doi.org/10.1016/j.jaad.2020.06.054.
19. Saskatchewan Formulary Bulletin. Bulletin #200. June 1, 2021. Update to the 62nd edition of the Saskatchewan formulary. Accessed on April 26, 2023. Available at: https://formulary.drugplan.ehealthsask.ca/Bulletins/Bulletin-0200-Jun-2021.pdf.
20. Manitoba Drug Benefits and Interchangeability Formulary Amendments. Bulletin #112. Accessed on April 26, 2023. Available at: https://www.gov.mb.ca/health/mdbif/docs/bulletins/bulletin112.pdf.
21. Ontario Ministry of Health. Exceptional Access Program Reimbursement Criteria for Frequently Requested Drugs. Accessed on April 26, 2023. Available at: https://www.health.gov.on.ca/en/pro/programs/drugs/docs/frequently_requested_drugs.pdf
22. RAMQ List of Medications. Updated April 13, 2023. Accessed on April 26, 2023. Available at: www.ramq.gouv.qc.ca/en/publications/citizens/legal-publications/Pages/list-medications.aspx.
23. New Brunswick Drug Plans Formulary Update. Published May 13, 2021. Accessed on April 26, 2023. Available at: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/NBDrugPlan/FormularyUpdates/NBDrugPlansBulletin-1053.pdf
24. Non-Insured Health Benefits First Nations and Inuit Health Branch. Drug Benefit List. Accessed on April 26, 2023. Available at: https://nihb.express-scripts.ca/NIHBProvider/benefits/pharmacy?page=drugbenefit-grid&benefit=pharmacy
25. Nova Scotia Formulary. Accessed on April 26, 2023. Available at: https://novascotia.ca/dhw/pharmacare/documents/formulary.pdf.
26. PEI Pharmacare Formulary. Accessed on April 26, 2023. Available at: https://www.princeedwardisland.ca/sites/default/files/publications/pei_pharmacare_formulary.pdf.
27. Government of Northwest Territories. Extended Health Benefits for Specific Disease Conditions Programs. Accessed on December 3, 2021. Available at: https://www.hss.gov.nt.ca/en/services/supplementary-health-benefits/extended-health-benefits-specified-disease-conditions.
28. Newfoundland and Labrador Prescription Drug Program. Special Authorization Drug Products. Updated on April 19, 2023. Accessed on April 26, 2023. Available at: https://www.gov.nl.ca/hcs/prescription/covered-specialauthdrugs/.
29. Saeki H, Nakahara T, et al. Journal of Dermatology 2016;43:1117–1145.
30. Balkrishnan R, et al. Arch Dis Child. 2003;88(5):423-7.
31. Eczema Society of Canada. Atopic Dermatitis: A Practical Guide to Management. Available at: https://eczemahelp.ca/wp-content/uploads/2019/03/ESC_AD_Practical-Guide-to-Management-for-HCP_2019.pdf. Accessed January 27, 2020.
DUPIXENT®, Sanofi and Freedom logos are trademarks of Sanofi, used under license by sanofi-aventis Canada Inc.
REGENERON® is a trademark of Regeneron Pharmaceuticals, Inc. All rights reserved.
© 2023 sanofi-aventis Canada Inc. All rights reserved.
MAT-CA-2300298
Last updated: 06/2023