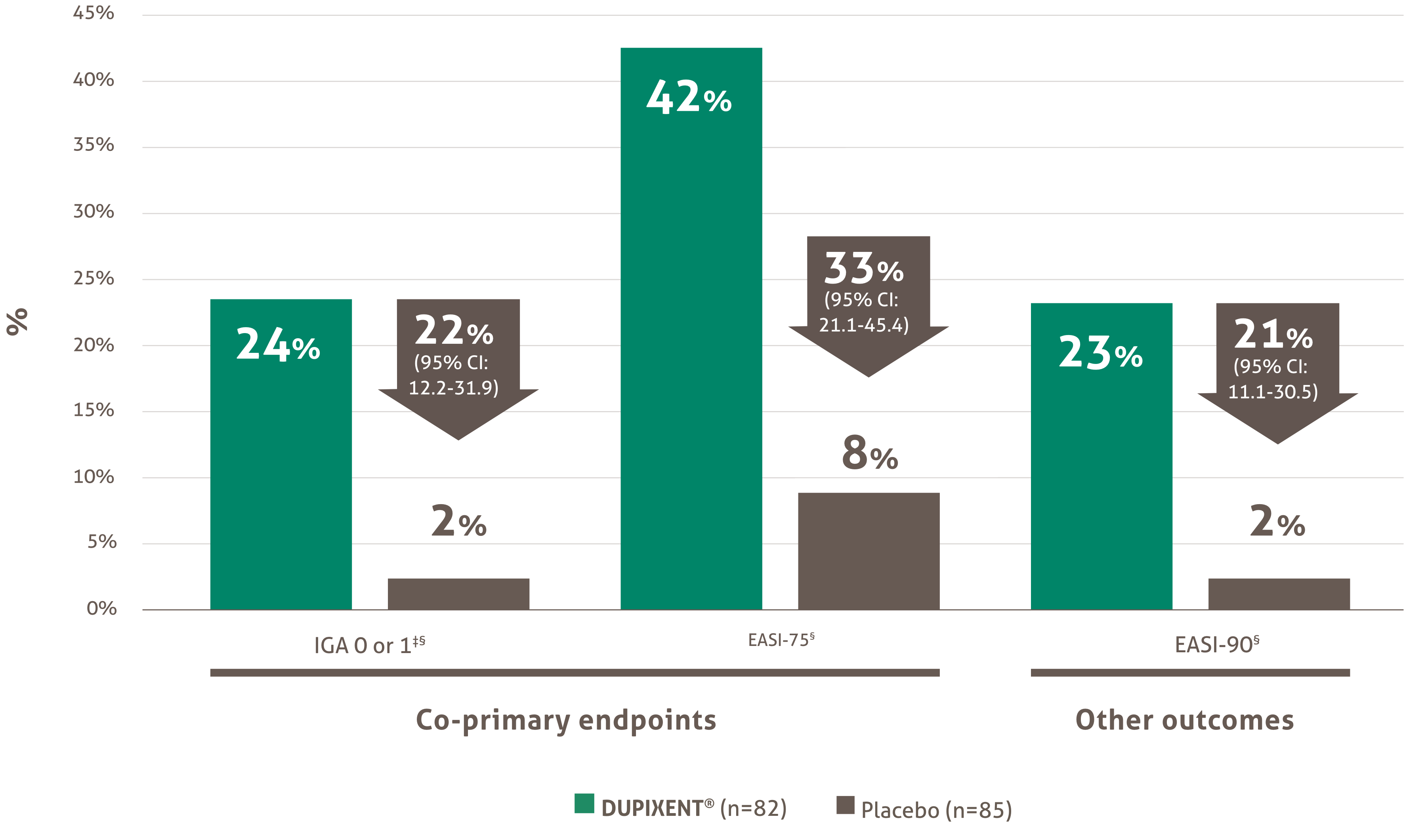
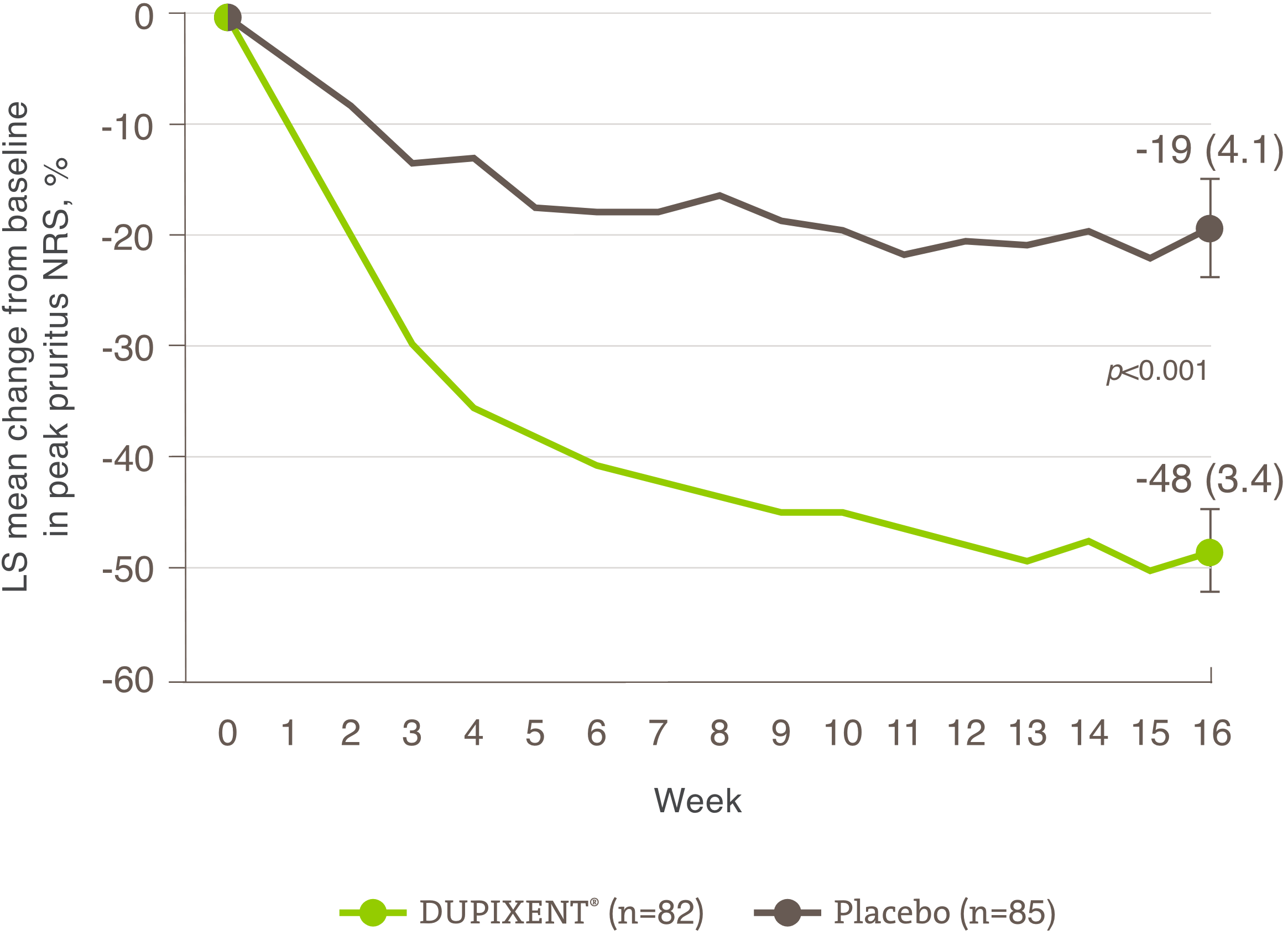
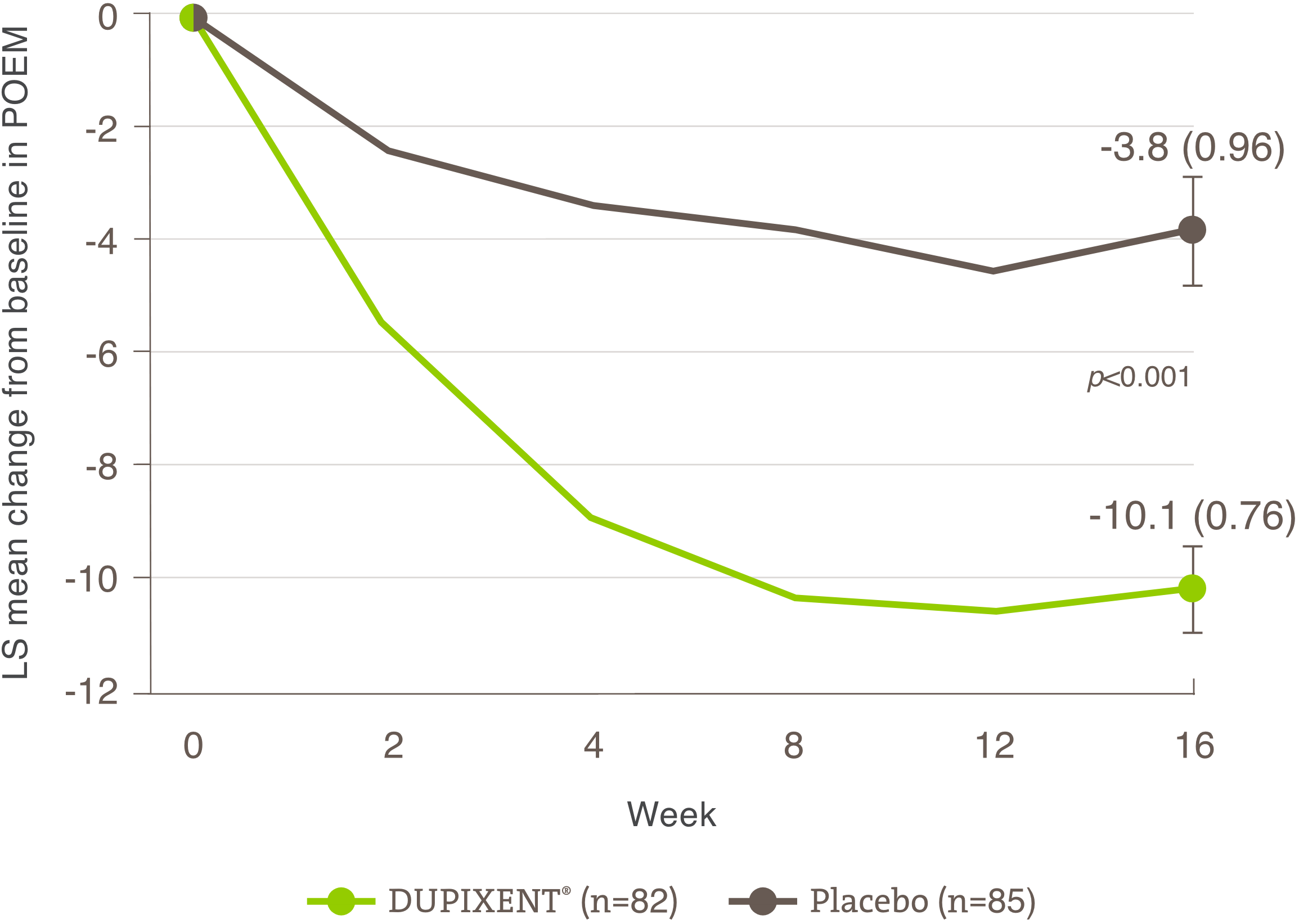
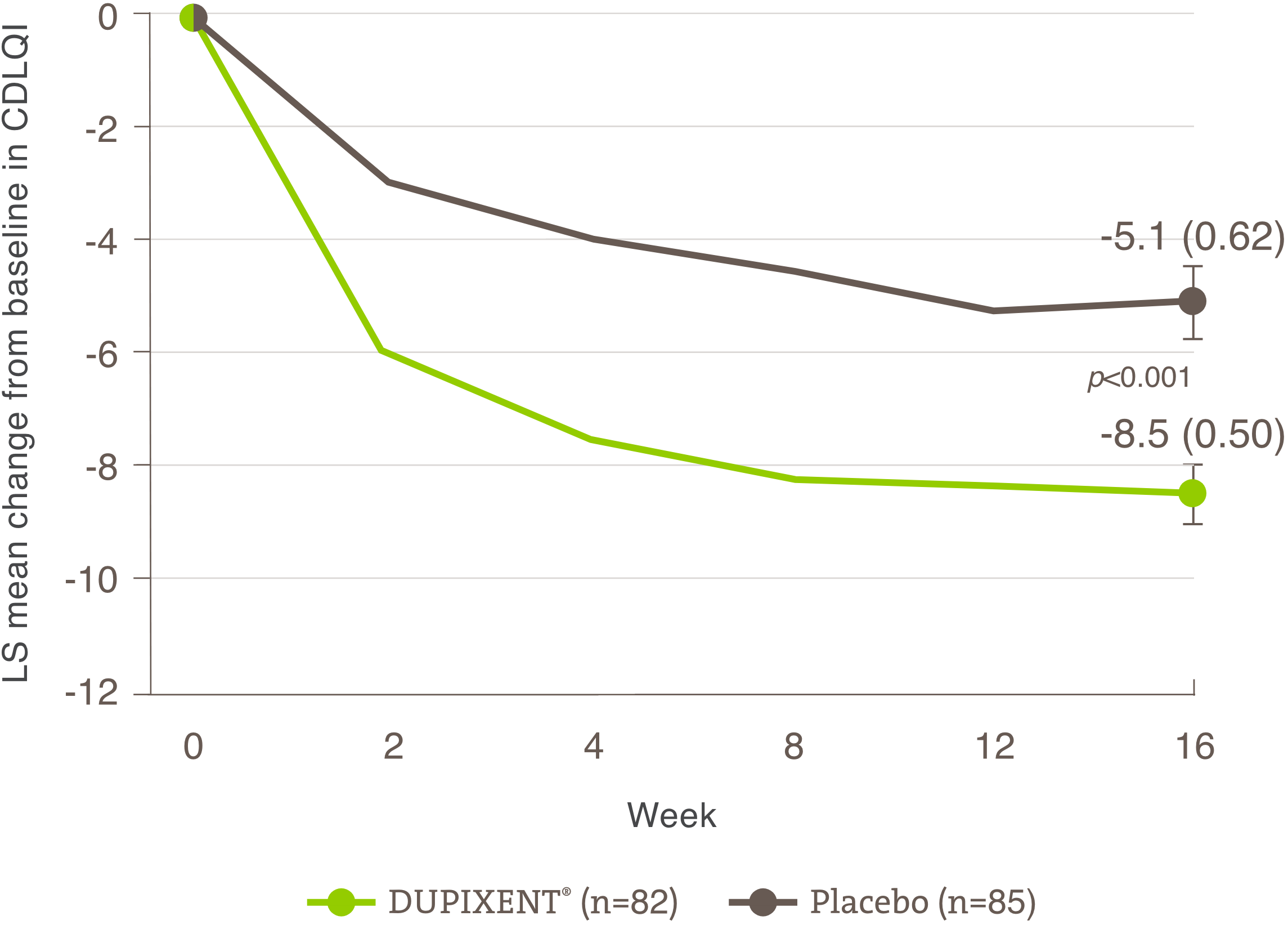
Significant improvement shown
in patient-reported outcomes (POEM and DLQI)
Demonstrated improvement in quality of life (measured by DLQI)
A larger proportion of patients treated with DUPIXENT® had ≥4 points improvement† in DLQI vs. placebo at week 16 (secondary endpoint):1
• SOLO 1 Trial: 64% DUPIXENT® (n=224) vs. 31% Placebo (n=224)
• SOLO 2 Trial: 73% DUPIXENT® (n=233) vs. 28% Placebo (n=236)
And at week 52 (secondary endpoint):1
• CHRONOS Trial: 80.0% DUPIXENT® (n=89) vs. 30.3% Placebo (n=264)
DLQI: Dermatology Life Quality Index
† A ≥4-point improvement corresponds to minimal clinically important difference.
Dermatology Life Quality Index (DLQI®)
DLQI is a patient self-assessment tool used to measure how atopic dermatitis has impacted quality of life over a one-week period. The following 10 questions are asked to the patients:12,13
1 |
Over the last week, how itchy, sore, painful or |
2 |
Over the last week, how embarrassed or self-conscious have you been because of your skin? |
3 |
Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? |
4 |
Over the last week, how much has your skin influenced the clothes you wear? |
5 |
Over the last week, how much has your skin affected any social or leisure activities? |
6 |
Over the last week, how much has your skin made it difficult for you to do any sport? |
7 |
Over the last week, has your skin prevented you from working or studying? If "No", over the last week how much has your skin been a problem at work or studying? |
8 |
Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? |
9 |
Over the last week, how much has your skin caused any sexual difficulties? |
10 |
Over the last week, how much of a problem has the treatment for your skin been, for example, by making your home messy, or by taking up time? |
|
0-1 |
No effect at all on patient’s life |
|
2-5 |
Small effect on patient’s life |
|
6-10 |
Moderate effect on patient’s life |
|
11-20 |
Very large effect on patient’s life |
|
21-30 |
Extremely large effect on patient’s life |
© A Y Finlay, G K Khan. April 1992. www.dermatology.org.uk. The above
permission does not affect the requirement for seeking of permission and of
possible payment when the DLQI is used for research or other purposes.
Step 1:
Patients fill out a 10-item questionnaire with each question scored on a 4-point scale:12 0=not at all; 1=a little; 2=a lot; 3=very much
Step 2:
Scores are summed to obtain a final score ranging from 0 to 30, where higher scores indicate a greater impairment of quality of life.12,13
Find out more about DLQI
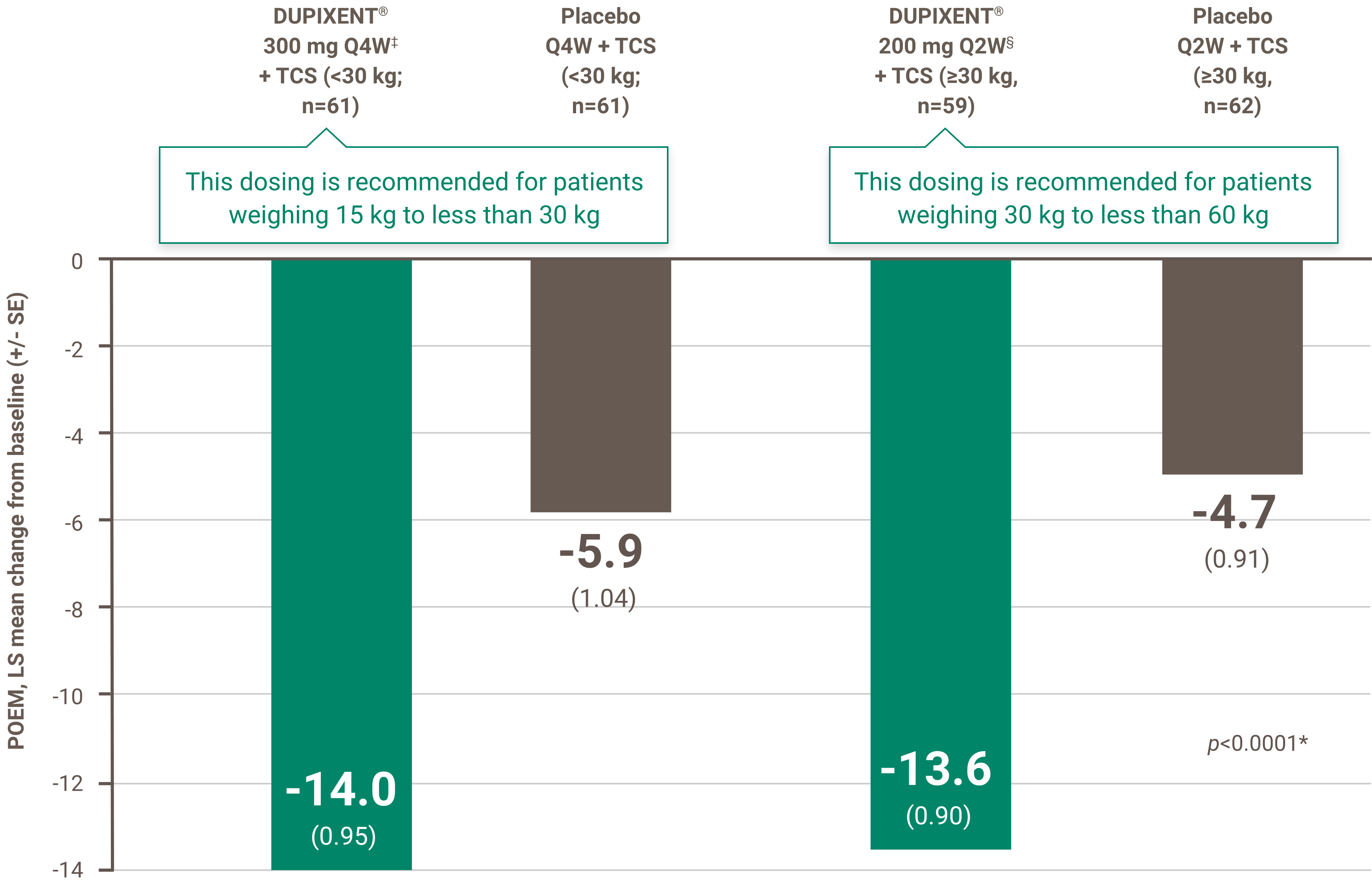
Demonstrated improvement in patient-reported outcomes on quality of life
A larger proportion of patients treated with DUPIXENT® had ≥4 points improvement† in POEM vs. placebo at week 16 (secondary endpoint):1
• SOLO 1 Trial: 68% DUPIXENT® (n=224) vs. 27% Placebo (n=224)
• SOLO 2 Trial: 72% DUPIXENT® (n=233) vs. 24% Placebo (n=236)
And at week 52 (secondary endpoint):1
• CHRONOS Trial: 76.4% DUPIXENT® (n=89) vs. 26.1% Placebo (n=264)
POEM: Patient Oriented Eczema Measure
† A ≥4-point improvement corresponds to minimal clinically important difference.
Patient Oriented Eczema Measure (POEM)
POEM is a patient-reported tool used to measure the severity of atopic dermatitis over one week. A total of 7 questions are asked in relation to:14
• Itchiness
• Bleeding
• Weeping or oozing
• Cracking
• Flaking
• Dryness/roughness
• Sleep disturbance
STEP 1:
Patients write down the number of days in the past week they were impacted by symptoms on a 5-point scale:15
0 = no days
1 = 1 to 2 days
2 = 3 to 4 days
3 = 5 to 6 days
4 = every day
STEP 2:
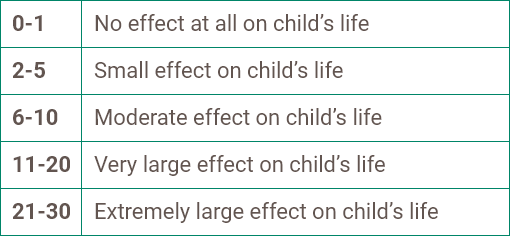
Scores are summed to obtain a final score ranging from 0 to 28, representing the patient’s severity of atopic dermatitis.15
0-2
Clear or
almost clear
3-7
Mild
eczema
8-16
Moderate
eczema
17-24
Severe
eczema
25-28
Very severe
eczema
© The University of Nottingham
Find out more about POEM
![]()
Dosing and Administration
Thinking about prescribing DUPIXENT®? Find helpful information to get started.
![]()
Connect With a Rep
Have questions about DUPIXENT®? Get answers from a representative.
DUPIXENT®, Sanofi and Freedom logos are trademarks of Sanofi, used under license by sanofi-aventis Canada Inc.
REGENERON® is a trademark of Regeneron Pharmaceuticals, Inc. All rights reserved.
© 2023 sanofi-aventis Canada Inc. All rights reserved.
MAT-CA-2300298
Last updated: 06/2023