DUPIXENT® is injected by subcutaneous injection and available as a pre-filled syringe and pen1
DUPIXENT® is intended for use under the guidance of a healthcare professional. A patient may self-inject DUPIXENT®, or the patient’s caregiver may administer DUPIXENT®. The DUPIXENT® pre-filled pen is only for use in adults and adolescents aged 12 years and older. Provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT® prior to use according to the Instructions for Use.
> In adolescents 12 years of age and older, it is recommended that DUPIXENT® be administered by, or under the supervision of, an adult.
Dosing: Adults (≥18 years) and Adolescents (12–17 years)
An initial dose of 400 mg (two 200 mg injections) followed by 200 mg given every other week for patients with severe asthma with a type 2/eosinophilic phenotype

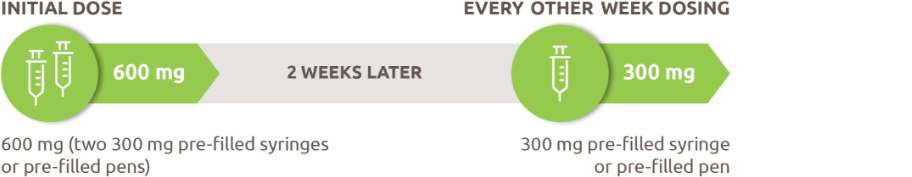
An initial dose of 600 mg (two 300 mg injections) followed by 300 mg given every other week for patients with OCS-dependent asthma or comorbid moderate-to-severe atopic dermatitis or comorbid severe CRSwNP
Dosing: Children (6 to 11 years of age)

* Based on population PK modelling.
For children (6 to 11 years of age) with asthma and comorbid moderate-to-severe AD, the recommended dose for AD in children and adolescents (6 to 17 years of age) should be followed. Please see the Product Monograph for more details.
A DUPIXENT® administration option for you and your patients
Features of the DUPIXENT® pre-filled pen include:
> Single-dose autoinjector
> Hidden needle
> Audible click when injection starts
> Window to check medicine and injection completion
> Step-by-step instructions for self-injection by subcutaneous injection
> Advise patients not to discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of DUPIXENT®. Reductions in corticosteroid dose, if appropriate, should be gradual and only performed under the supervision of a healthcare professional.1
> Advise atopic dermatitis or CRSwNP patients with comorbid asthma not to adjust or stop their asthma treatments without consulting their healthcare professional.1
Preparation for use1
- Before injection, instruct patients and/or caregivers to remove the DUPIXENT® pre-filled syringe or pre-filled pen from the refrigerator.
- 300 mg pre-filled syringe with a needle shield, pre-filled syringe, or pre-filled pen: allow DUPIXENT® to reach room temperature by waiting 45 minutes.
- 200 mg pre-filled syringe with a needle shield or pre-filled pen: allow DUPIXENT® to reach room temperature by waiting 30 minutes.
- Patients and/or caregivers should inspect DUPIXENT® for particulate matter and discoloration prior to administration.
- Patients and/or caregivers should not use the pre-filled syringe/pen if the liquid contains visible particulate matter, or is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow).
- Patients and/or caregivers should discard any unused product remaining in the pre-filled syringe/pen in accordance with local requirements.

Important administration instructions1
- Patients and/or caregivers should read the Instructions for Use prior to injecting.
- Instruct patients and/or caregivers to administer subcutaneous injection into the thigh or abdomen, except for the 2 inches (5 cm) around the navel.
- The upper arm can also be used if a caregiver administers the injection.
- It is important to rotate the injection site with each injection. DO NOT inject DUPIXENT® into skin that is tender, damaged, bruised, or scarred. DO NOT inject through clothes.
- Choose a different injection site for each DUPIXENT® injection. If you need a second injection to complete your dose then leave at least 5 cm (2 inches) between the two injection sites.
Invite your patients to watch our injection training videos so they can see how to inject DUPIXENT®.
Self-injection
For caregivers
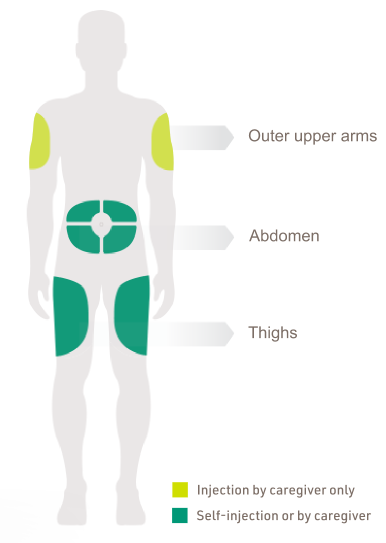
The DUPIXENT® pre-filled pen is only for use in adults and adolescents aged 12 years and older. In adolescents 12 years of age and older, it is recommended that DUPIXENT® be given by or under the supervision of an adult. The DUPIXENT® pre-filled syringe should be given by a caregiver in patients under 12 years of age, the DUPIXENT® pre-filled syringe is the presentation appropriate for this population.
Storage and Handling1

DUPIXENT® should be refrigerated at 2–8°C, never frozen or exposed to extreme heat

DUPIXENT® is light sensitive, so it should be kept in its carton in order to protect it from light

Do not shake DUPIXENT® or use beyond the expiration date

DUPIXENT® may be kept at room temperature (up to 25°C) for up to 14 days
After removal from the refrigerator, DUPIXENT® must be used within 14 days or discarded.
CRSwNP=chronic rhinosinusitis with nasal polyposis; OCS=oral corticosteroid; AD=atopic dermatitis.
![]()
Connect With a Rep
Have questions about DUPIXENT®? Get answers from a representative.

Freedom Support Program
Find out what the Freedom Support Program can bring to your patients and how you can help them access it.
DUPIXENT®, Sanofi and Freedom logos are trademarks of Sanofi, used under license by sanofi-aventis Canada Inc.
REGENERON® is a trademark of Regeneron Pharmaceuticals, Inc. All rights reserved.
© 2023 sanofi-aventis Canada Inc. All rights reserved.
MAT-CA-2300228
Last updated: 06/2023




